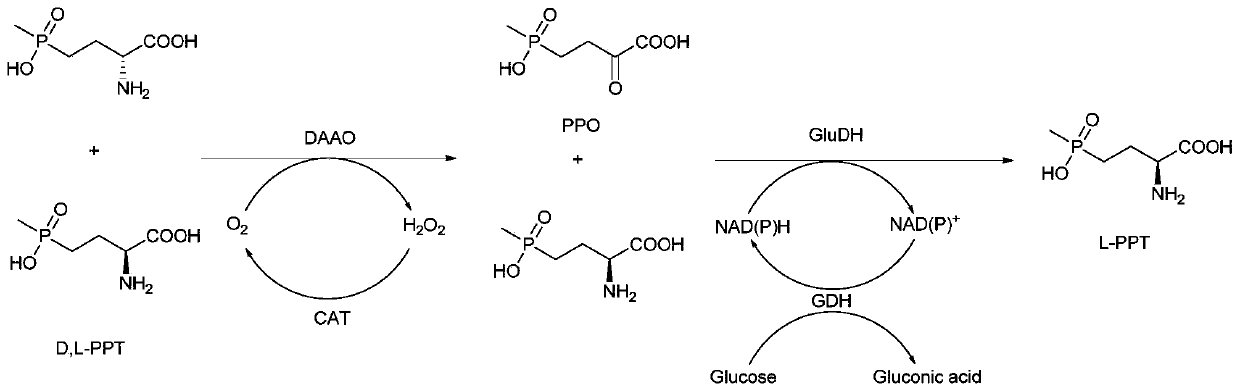
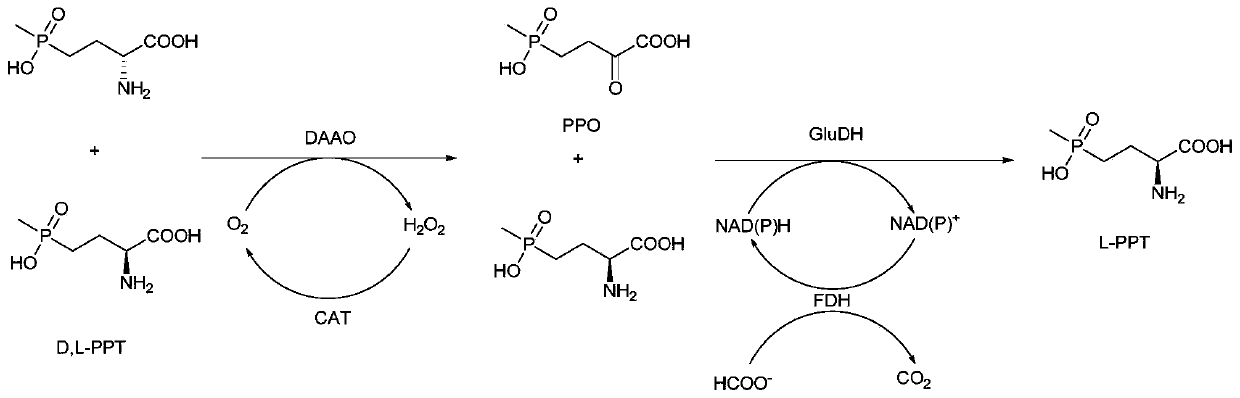
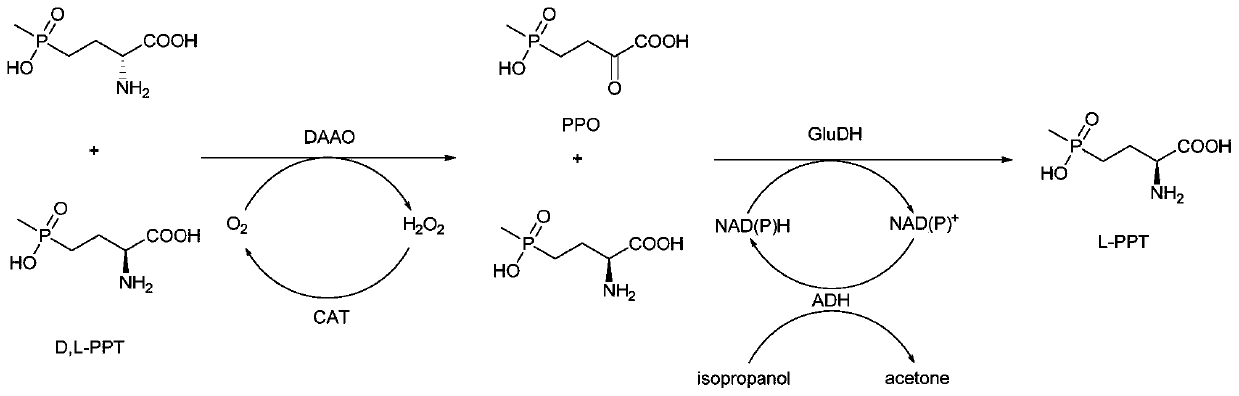
Method for preparing L-phosphinothricin through de-racemization by biological enzyme method, phosphinothricin dehydrogenase mutant and application
A biological enzyme method, glufosinate-ammonium technology, applied in the biological field, can solve the problems of complex process, waste of raw materials, expensive chiral resolution reagents, etc., and achieve the effect of good catalytic efficiency and yield improvement.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 1. The cultivation of engineered bacteria
[0042] After the engineering bacteria were activated by streaking on a plate, a single colony was picked and inoculated into 10 mL LB liquid medium containing 50 μg / mL kanamycin, and cultured with shaking at 37 °C for 10 h. Transfer to 50mL LB liquid medium also containing 50μg / mL kanamycin according to 2% inoculum amount, and culture with shaking at 37°C until OD 600 When it reaches about 0.8, add IPTG with a final concentration of 0.5mM, and culture with shaking at 28°C for 12h. After the cultivation, the culture solution was centrifuged at 8000rpm for 10min, the supernatant was discarded, the bacteria were collected, and stored in a -80°C ultra-low temperature refrigerator until use.
[0043] 2. Preparation of crude enzyme solution
[0044] Wash the collected cells with pH 8 phosphate buffer (50 mM pH=8 phosphate buffer) twice, then resuspend the cells in pH 8 phosphate buffer (50 mM) Cells were ultrasonically broken 30 ...
Embodiment 2
[0049] Determination of specific enzyme activity of glufosinate-ammonium dehydrogenase and its mutants.
[0050] Enzyme activity unit (U) is defined as: under the conditions of 35°C and pH 7.4, the amount of enzyme required to generate 1 μmol of L-glufosinate-ammonium per minute is defined as an enzyme activity unit, U. Specific enzyme activity is defined as the number of activity units per mg of enzyme protein, U / mg.
[0051] Standard conditions for enzyme activity detection: 100mM 2-carbonyl-4-(hydroxymethylphosphinyl)-butyric acid, 10mM NADPH, appropriate amount of enzyme solution, 30°C, pH 7.4, 600 rpm for 10 minutes, sample treatment And carry out HPLC detection analysis.
[0052] Protein concentration was determined with BCA protein assay kit (Nanjing KGI Biotechnology Development Co., Ltd., Nanjing).
Embodiment 3
[0054] Construction and screening of glufosinate-ammonium dehydrogenase mutant library.
[0055] 1. Construction of genetically engineered bacteria
[0056] The gene sequence of glufosinate-ammonium dehydrogenase (GenBank No.: WP_101496154) derived from Thiopseudomonas denitrificans was codon-optimized and sent to Shenggong Bioengineering (Shanghai) Co., Ltd. for whole gene synthesis. and cloned into the recombinant expression plasmid pETduet-1 to construct the plasmid pETduet-1-GluDH. After the recombinant plasmid was verified to be correct by sequencing, it was transferred into the expression host E. coli BL21 (DE3) for subsequent expression of the recombinant glufosinate-ammonium dehydrogenase. The codon-optimized glufosinate-ammonium dehydrogenase gene sequence is shown in SEQ ID No.1, and the amino acid sequence is shown in SEQ ID No.2.
[0057] 2. Construction of glufosinate-ammonium dehydrogenase mutant library
[0058] The first step is to construct a glufosinate-am...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com