A polymer solid electrolyte based on the principle of bulk plasticization and its preparation method
A solid electrolyte and polymer technology, applied in solid electrolytes, non-aqueous electrolytes, circuits, etc., can solve the problems of low ionic conductivity at room temperature, difficult to guarantee compatibility, poor stability, etc. Strong ability, good high temperature mechanical strength effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
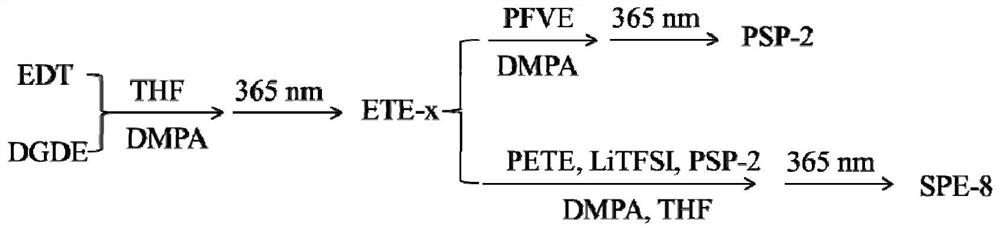
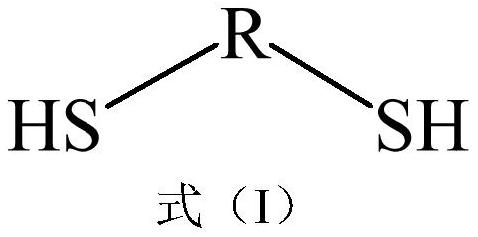
[0039] Example 1: Preparation of polyether sulfide bulk plasticizer (PSP-1). 1,2-ethanedithiol (EDT, formula I, R: C2) and diethylene glycol divinyl ether (DGDE, formula II (f)) are reactive monomers, vinyl ether (EVE, formula III ( b)) is an end-capping agent, 2,2-dimethoxy-2-phenylacetophenone (DMPA) is a photoinitiator, and anhydrous tetrahydrofuran (THF) is a solvent.
[0040] The following operations were all carried out in an argon atmosphere glove box and were divided into two steps. Step 1: Add EDT and DGDE according to a certain ratio in a 20mL small glass bottle, add an appropriate amount of THF, then add DMPA, stir in the dark to dissolve completely, and trigger the reaction under 365nm ultraviolet light to prepare sulfhydryl-terminated Linear polyether sulfide (ETE-x); the second step: take out part of ETE-x, add the end-capping agent EVE and an appropriate amount of DMPA, stir in the dark to completely dissolve, and initiate the reaction under 365nm ultraviolet l...
Embodiment 2
[0044] Example 2: Preparation of polyether sulfide bulk plasticizer (PSP-2). EDT and DGDE are reactive monomers, propyl cellulose vinyl ether (PFVE, formula III(i)) is an end-capping agent, DMPA is a photoinitiator, and anhydrous THF is a solvent.
[0045] Except that the end-capping agent was changed, the dosage and operation steps of each reagent were the same as in Example 1.
[0046] The code number of the polyether sulfide bulk plasticizer sample is: PSP-2.
Embodiment 3
[0048] Example 3: Preparation of bulk plasticized polymer solid electrolyte (SPE-1): triallylamine (TAA, formula IV (e)) is a crosslinking agent, PSP-1 is a plasticizer, and bistrifluorosulfone Lithium imide (LiTFSI, formula VI(a)) is a small molecule lithium salt, DMPA is used as a photoinitiator, and anhydrous THF is used as a solvent.
[0049] The following operations are all carried out in a glove box. Add ETE-x, TAA, PSP-1, LiTFSI and DMPA to a 20mL small glass bottle according to a certain ratio, add an appropriate amount of THF and stir it in the dark to dissolve completely, and use a spatula to apply it on the BOPP material substrate and irradiated under 365nm ultraviolet light, and the solvent was evaporated at room temperature.
[0050] In the ETE-x, x is preferably 10-20.
[0051] The molar ratio of ETE-x to TAA is 3:2.
[0052] The molar ratio of the EO / ES segment to the lithium salt is: [EO / ES] / Li + =10 / 1.
[0053] The total mass percentage of the PSP-1 in the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com