Phosphate ester oligomer natural gas drag reducer and synthesis method and application thereof
A synthesis method and oligomer technology, applied in phosphorus organic compounds, chemical instruments and methods, gas/liquid distribution and storage, etc., can solve the problem of insignificant drag reduction effect, inability to apply natural gas pipeline drag reduction on a large scale, and scope of application Restrictions and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
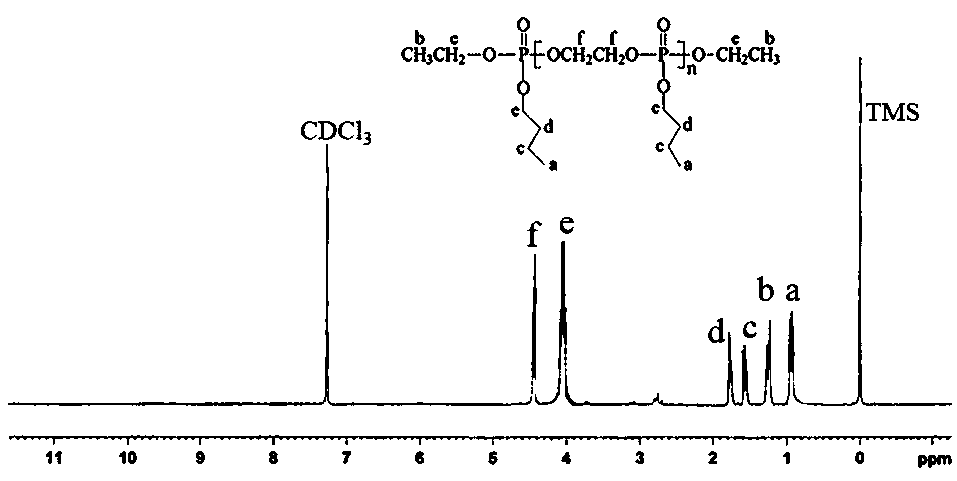
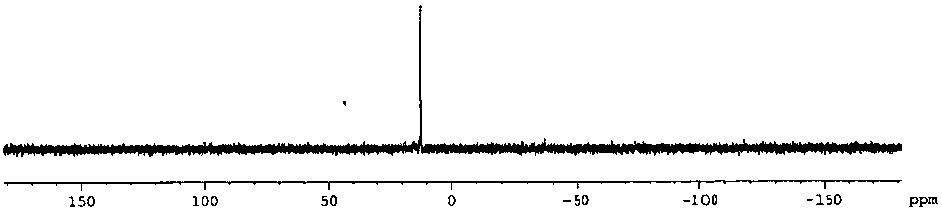
[0044] Add 153.3g of phosphorus oxychloride, 1.33g of anhydrous aluminum trichloride and 600mL of methyl ethyl ketone into a 3000mL four-necked bottle equipped with a reflux condenser, a thermometer, a stirrer, and a constant pressure dropping funnel, and cool the four-necked bottle to - 10°C and maintained for 20min, slowly drop 74.1g of n-butanol under stirring, and control the temperature of the system not to exceed 0°C. And react at -5~5°C for 2h. Raise the temperature to 20°C and react for 2h. Slowly add 62.1 g of ethylene glycol dropwise at 20°C, control the system temperature not to exceed 30°C, and react at 30°C for 2 hours, then raise the temperature to 50°C, and react for 4 hours. Add 9.2 g of ethanol at 50° C., and reflux for 4 hours. The reaction ends when the pH value of the solution is close to neutral. The crude product was washed with water and the solvent was removed to obtain 161.3 g of light yellow viscous liquid with a yield of 89.6 wt%.
[0045] The str...
Embodiment 2
[0058] Add 153.3g of phosphorus oxychloride, 1.9g of anhydrous magnesium chloride and 600mL of butyl ether into a 3000mL four-necked flask equipped with a reflux condenser, thermometer, stirrer, and constant pressure dropping funnel, and cool the four-necked flask to -10°C And keep it for 20 minutes, slowly drop 130.2g of n-octanol under stirring, control the temperature of the system not to exceed 0°C, and react at -5~5°C for 3h. Raise the temperature to 25°C and react for 4h. Slowly add 118.2g butyl ether solution of hexanediol dropwise at 25°C, control the temperature of the system not to exceed 30°C, and react at 30°C for 3h, raise the temperature to 50°C, and react for 8h. 23.2 g of n-heptanol was added at 50° C., and refluxed for 6 hours. The reaction ended when the pH of the solution was close to neutral. The crude product was washed with water and the solvent was removed to obtain 257.0 g of light yellow viscous liquid with a yield of 87.7 wt%.
[0059] The structura...
Embodiment 3
[0064]Add 153.3g of phosphorus oxychloride, 7.9g of pyridine and 600mL of 2-pentanone into a 3000mL four-necked flask equipped with a reflux condenser, thermometer, stirrer, and constant pressure dropping funnel, cool the four-necked flask to -10°C and Maintain for 20 minutes, slowly drop 102.2 g of n-hexanol under stirring, control the temperature of the system not to exceed 0°C, and react at -5~5°C for 3 hours. Raise the temperature to 30°C and react for 4h. Slowly add 104.2 g of 1,5-pentanediol dropwise at 30°C, control the system temperature to about 30°C, and react for 4 hours; raise the temperature to 60°C, and react for 7 hours. 14.8 g of n-butanol was added at 60° C., and refluxed for 5 hours. The reaction ended when the pH value of the solution was close to neutral. The crude product was washed with water and the solvent was removed to obtain 222.3 g of a light yellow viscous liquid with a yield of 88.9 wt%.
[0065] The structural formula of the product obtained in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com