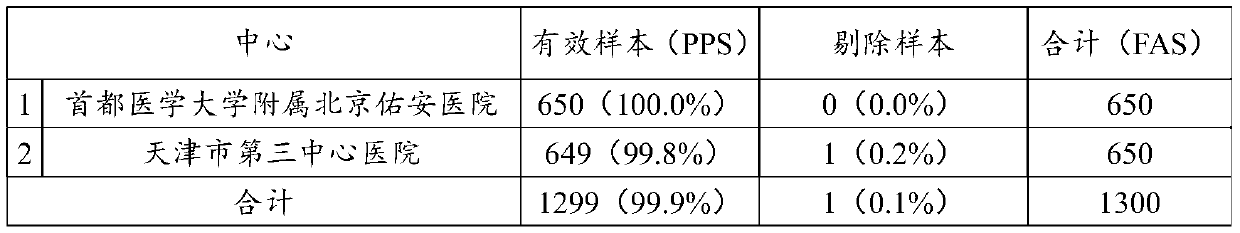
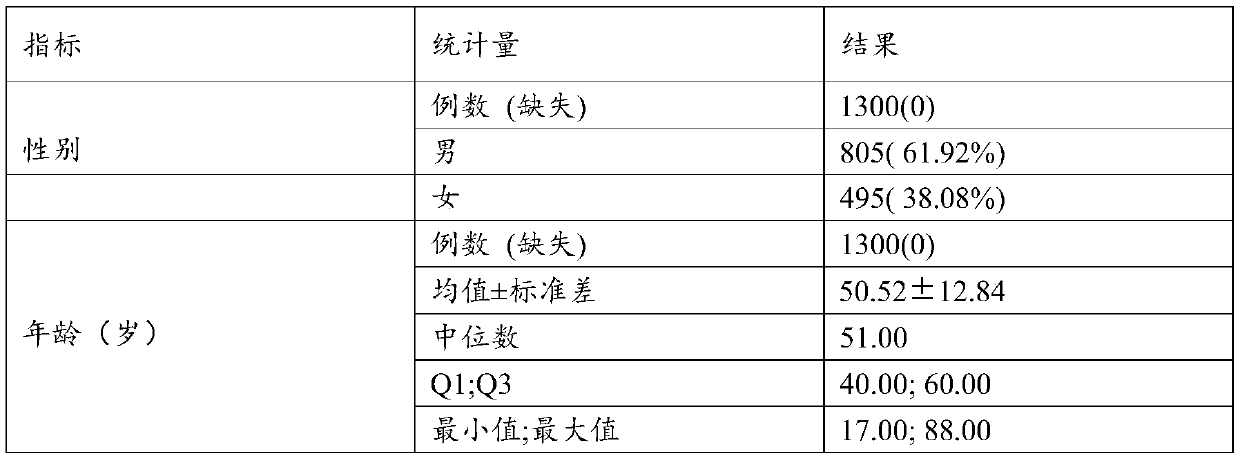
Golgi apparatus protein 73 monoclonal antibody, kit and application
A technology of monoclonal antibody and Golgi apparatus, applied in anti-animal/human immunoglobulin, biological testing, biochemical equipment and methods, etc., can solve the unfavorable early diagnosis, specificity and sensitivity of clinical diagnosis of liver cirrhosis Not advanced question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] This example provides a monoclonal antibody stock solution of Golgi protein 73.
[0027] The antibody stock solution is obtained by injecting the preserved hybridoma cells of the present invention in Balb / c mice to prepare ascites, and then purifying Protein G.
[0028] Specifically, ascitic fluid was prepared by injecting hybridoma cells with a deposit number of CGMCC No. 19282 in Balb / c mice, and then protein G was purified to obtain GP73 monoclonal antibody stock solution-1. Ascites was prepared by injecting hybridoma cells with the deposit number CGMCC No. 19283 in Balb / c mice, and then protein G was purified to obtain GP73 monoclonal antibody stock solution-2.
Embodiment 2
[0030] This embodiment provides a kit for detecting the level of GP73 in human serum. The specification of the kit is 96 servings / box, which contains the following components:
[0031] (1) GP73 antibody-coated plate (in each kit, 96-well plate × 1)
[0032] The coated plate is prepared by the following method: coat the GP73 monoclonal antibody stock solution-1 provided in Example 1 at a concentration of 2 μg / ml on a 96-well microtiter plate, and incubate at 2-8°C for no less than 16 hours ; Shake off the coating solution in the microplate, wash it once with phosphate buffer, add blocking solution, and incubate at 2-8°C for no less than 16 hours; shake off the blocking solution in the microplate, dry it in Store at 2-8°C.
[0033] (2) GP73 calibrator (in each kit, 0.5mL×8 bottles)
[0034] The GP73 calibrator is obtained by expressing recombinant GP73 protein (referred to as GP73 antigen) in Escherichia coli by genetic engineering methods, and then adding 0.02M phosphate buf...
Embodiment 3
[0043] This example provides a method for detecting human serum GP73 levels using the kit provided in Example 2.
[0044] 1. Sample collection: Collect venous blood and let it stand for more than half an hour. After it is fully coagulated, centrifuge at 1500rpm for 5-10min, and take the upper serum.
[0045] In order to ensure accurate detection results, the present invention requires the use of fresh samples as much as possible to avoid repeated freezing and thawing; fresh samples can be stored for 7 days at 2-8°C, and the storage period at -20°C is no more than 3 months. At -80°C, no more than 2 years; samples must be transported under cryopreservation conditions and comply with relevant biosafety regulations; samples with severe hemolysis, hyperlipidemia or serious contamination cannot be used; samples cannot use sodium azide as a preservative. If the sample has been frozen, it should be equilibrated at room temperature (18-28°C) for at least 30 minutes before testing.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com