Preparation method of nano copper particles for treating inflammatory diseases
An inflammatory disease, nano-copper technology, applied in the field of medicine, can solve the problems of glomerular filtration membrane excretion, low catalytic efficiency, small specific surface area, etc., achieve super broad-spectrum antioxidant performance, mild conditions, The effect of simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
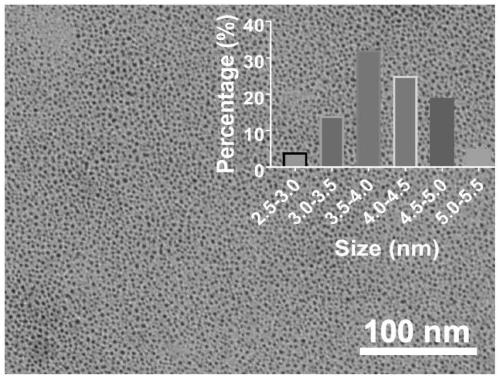
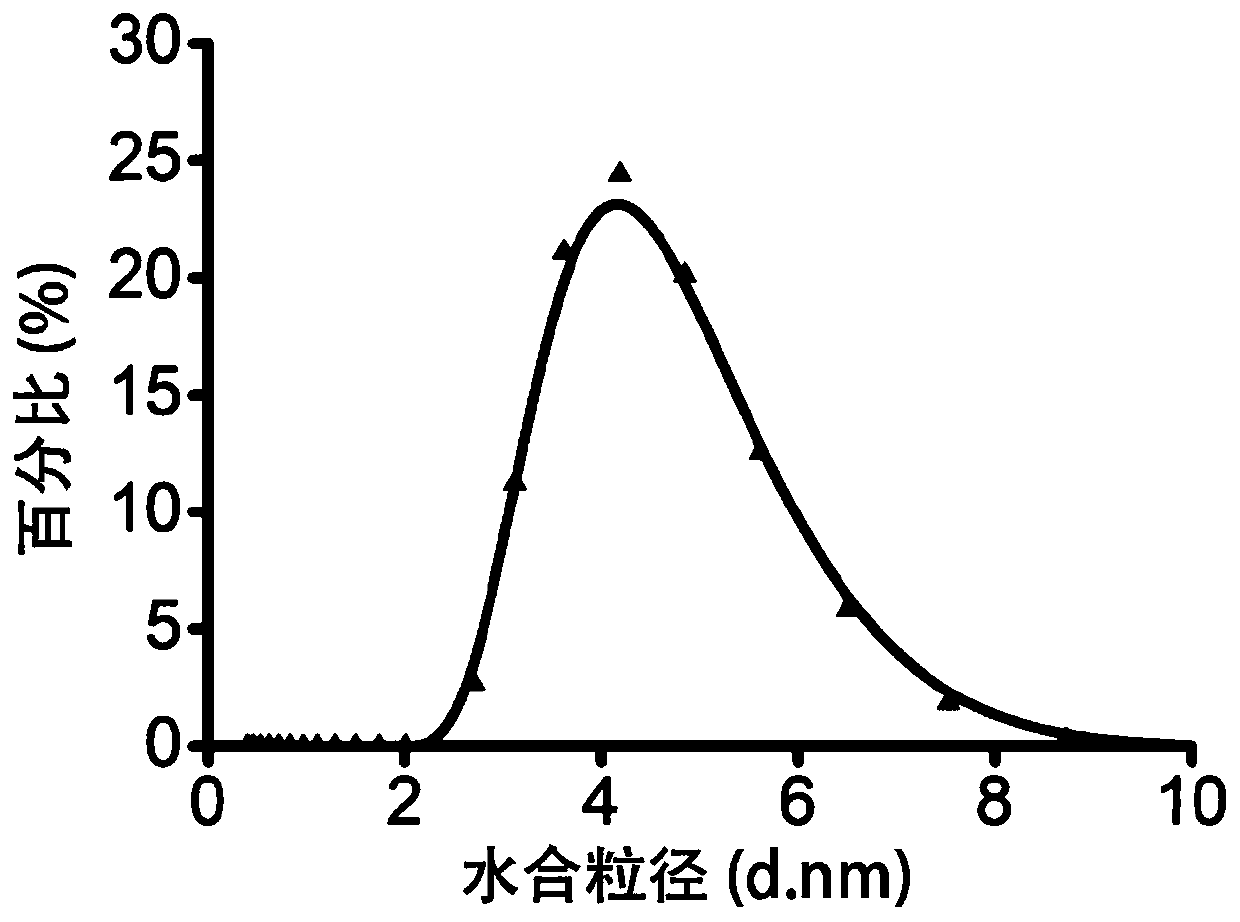
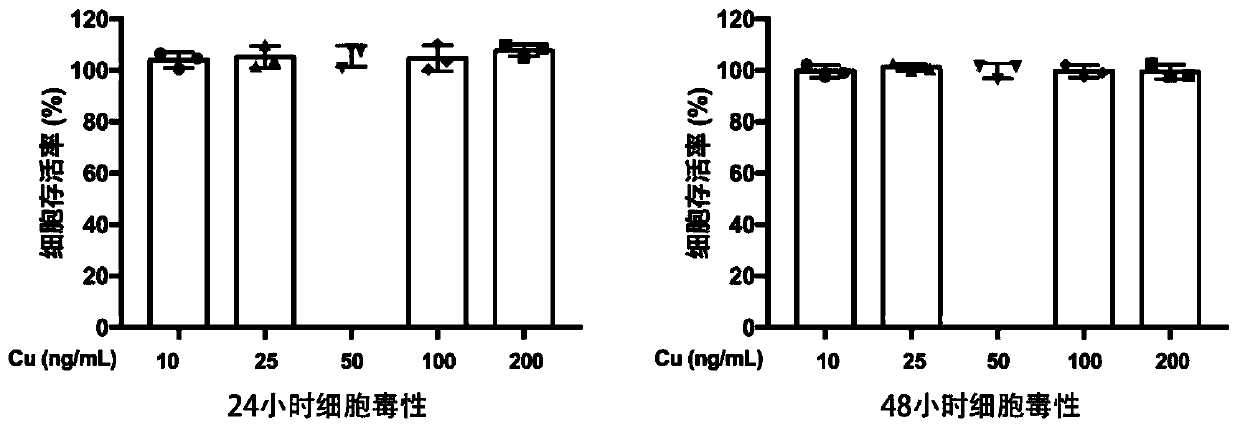
Image
Examples
Embodiment 1
[0047] Example 1 Preparation of Nano Copper Particles for Treatment of Inflammatory Diseases
[0048] The preparation process is as follows:
[0049] 1) Weigh 0.0672g of anhydrous copper chloride, add it to 50mL of deionized water, stir it magnetically for 10 minutes to fully dissolve it, prepare a copper chloride solution with a molar concentration of 10mM, and place it in an oil bath at 80°C;
[0050] 2) Weigh 0.8806g of ascorbic acid, add it into 50mL of deionized water, and fully dissolve it to form an ascorbic acid solution with a molar concentration of 0.1M;
[0051] 3) Under magnetic stirring, slowly drop the ascorbic acid solution obtained in step 2) into the round-bottomed flask containing the copper chloride solution obtained in step 1 through the dropping funnel, the dripping speed is 3 seconds / drop, 80 ℃ constant temperature oil Under the condition of the bath, stir magnetically for 10 hours, and finally obtain a brown-yellow to brown-black suspension C;
[0052]...
Embodiment 2
[0052] 4) Centrifuge the suspension C obtained in step 3), the centrifugation speed is 8000 rpm, and the centrifugation time is 10 minutes, discard the precipitate, dialyze the supernatant with a dialysis bag with a molecular weight of 3500D for 48 hours, freeze Dry for 24 hours to obtain ultra-small diameter nano-copper particle powder with strong oxidation resistance. Example 2 Preparation of Nano Copper Particles for Treatment of Inflammatory Diseases
[0053] The preparation process is as follows:
[0054] 1) Weigh 0.0672g of anhydrous copper chloride, add it to 50mL of deionized water, stir it magnetically for 10 minutes to fully dissolve it, prepare a copper chloride solution with a molar concentration of 10mM, and place it in an oil bath at 80°C;
[0055] 2) Weigh 1.7612g of ascorbic acid, add it into 50mL of deionized water, and fully dissolve it to form an ascorbic acid solution with a molar concentration of 0.2M;
[0056] 3) Under magnetic stirring, slowly drop the...
Embodiment 3
[0057] 4) Centrifuge the suspension C obtained in step 3), the centrifugation speed is 8000 rpm, and the centrifugation time is 10 minutes, discard the precipitate, dialyze the supernatant with a dialysis bag with a molecular weight of 3500D for 48 hours, freeze Dry for 24 hours to obtain ultra-small diameter nano-copper particle powder with strong oxidation resistance. Example 3 Preparation of Nano Copper Particles for Treatment of Inflammatory Diseases
[0058] The preparation process is as follows:
[0059] 1) Weigh 0.0672g of anhydrous copper chloride, add it to 50mL of deionized water, stir it magnetically for 10 minutes to fully dissolve it, prepare a copper chloride solution with a molar concentration of 10mM, and place it in an oil bath at 80°C;
[0060] 2) Weigh 3.5224g of ascorbic acid, add it to 50mL of deionized water, and dissolve it fully to form an ascorbic acid solution with a molar concentration of 0.4M;
[0061] 3) Under magnetic stirring, slowly drop the a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com