Water-soluble composite immunoadjuvant and application thereof
A water-soluble composite and immune adjuvant technology, applied in vaccines, antiviral agents, multivalent vaccines, etc., can solve problems such as frequent stress responses of animals, reduce animal growth speed, increase veterinary workload, etc., and achieve tissue damage Effects of operations related to less stress response, extended storage time, and ease of mass production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
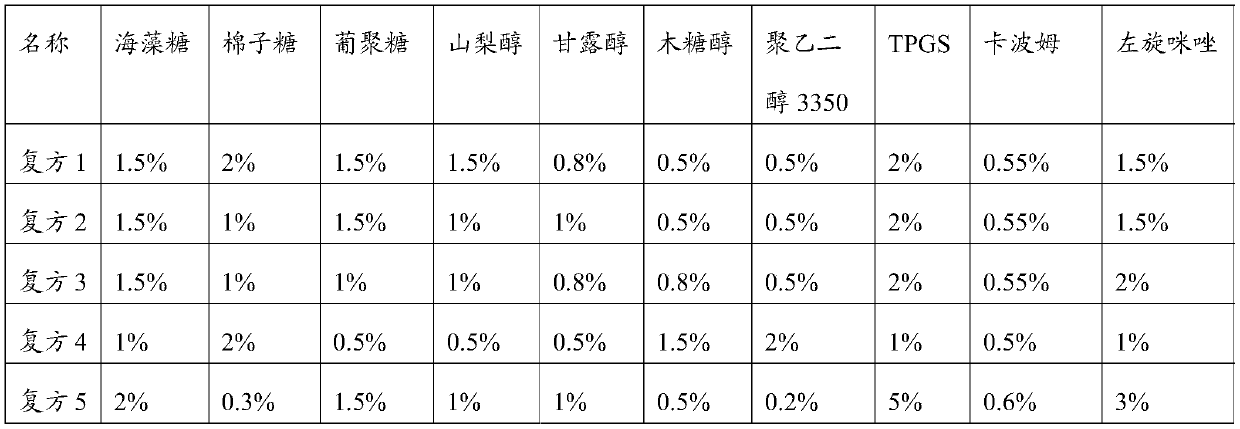
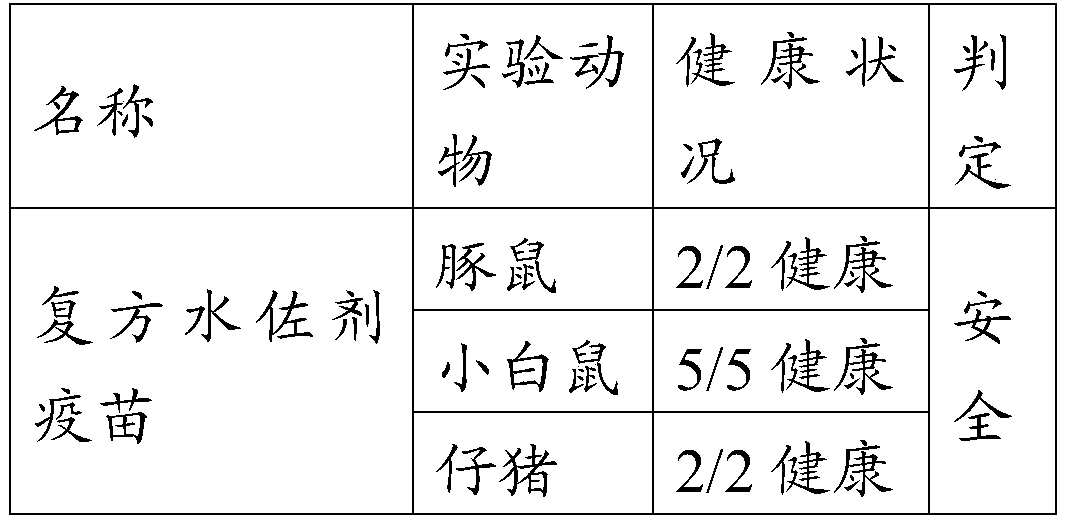
Examples
Embodiment 1
[0026] The preparation of embodiment 1 foot-and-mouth disease vaccine of the present invention
[0027] 1 Preparation of inactivated antigen
[0028] 1.1 Preparation of virus solution
[0029] 1.1.1 Preparation of type A foot-and-mouth disease virus liquid
[0030] 10000L bioreactor full suspension culture of BHK-21 cells, the cell density reaches 3-5×10 6 Inoculation of foot-and-mouth disease virus cell-adapted strain (bovine foot-and-mouth disease virus A / AKT-Ⅲ strain, prepared by Inner Mongolia Biwei Antai Biotechnology Co., Ltd.) according to the virus multiplicity of infection MOI 0.01-0.1, and the virus stock solution was prepared, and the stirring speed did not exceed 40rpm , cultivated for 8-12h to harvest the virus liquid.
[0031] 1.1.2 Preparation of O-type foot-and-mouth disease virus liquid
[0032] 10000L bioreactor full suspension culture of BHK-21 cells, the cell density reaches 3-5×10 6 When each / ml, inoculate foot-and-mouth disease virus cell-adapted strai...
Embodiment 2
[0060] Example 2 Foot-and-mouth disease compound water adjuvant inactivated vaccine efficacy detection
[0061] With 6-month-old FMDV antibody-negative calves, 10 calves were intramuscularly injected with 2 mL of A / AKT-Ⅲ strain foot-and-mouth disease compound water adjuvant inactivated vaccine in the neck, and blood was collected intravenously on the 28th day. ELISA test is used to judge the protectiveness of the vaccine by measuring the antibody titer.
[0062] Use about 40kg, 10 pigs that are negative for FMDV antibody, intramuscularly inject 2mL of O / Mya98 / XJ / 2010 strain foot-and-mouth disease compound water adjuvant inactivated vaccine behind each ear, and collect blood intravenously on the 21st day, and its serum is subjected to liquid phase resistance. ELISA test was used to judge the protection by measuring the antibody titer. The FMDV antibody titers of both pigs and cattle were greater than 1:128, indicating 99% protection.
Embodiment 3
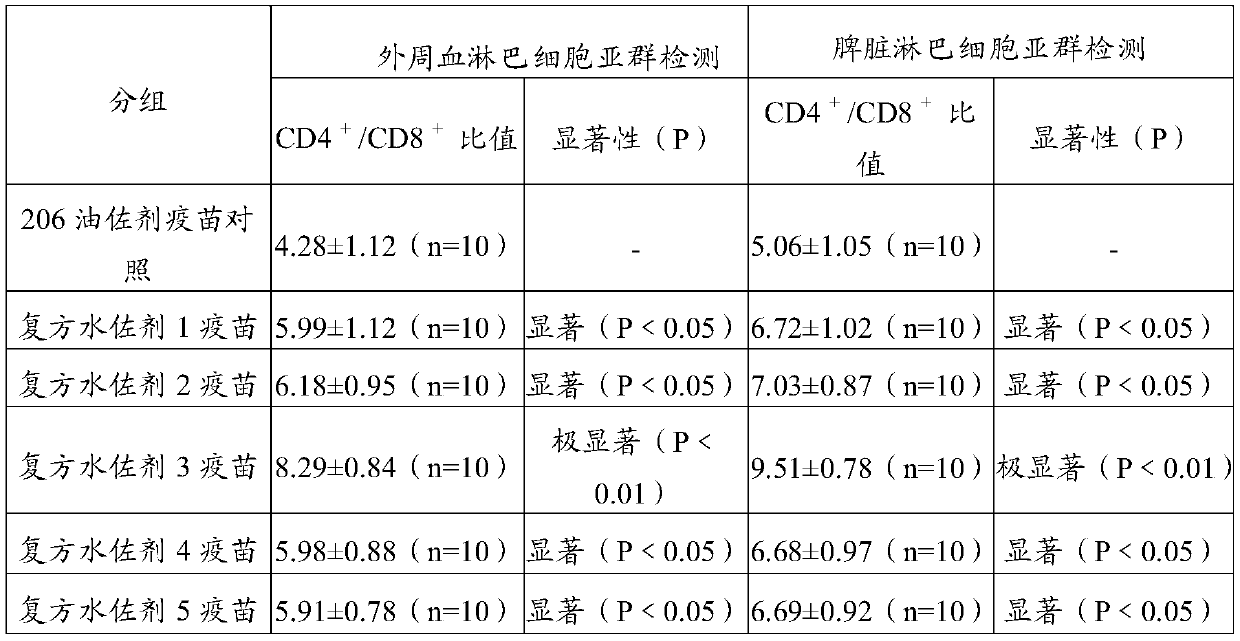
[0063] Embodiment 3 The immune effect monitoring of the foot-and-mouth disease compound water adjuvant vaccine of the present invention
[0064] The diluted foot-and-mouth disease inactivated antigen and 206 adjuvant are mixed and emulsified according to the ratio of (V / V) 46:54 to prepare the foot-and-mouth disease oil adjuvant vaccine. Reference can be made to the patent application (Application No. 201510236068.4) titled "A Preparation Method for Foot-and-Mouth Disease Vaccine".
[0065] The O-type foot-and-mouth disease oil adjuvant vaccine (O / Mya98 / XJ / 2010 strain) prepared and the O-type foot-and-mouth disease compound water adjuvant inactivated vaccine prepared by 2.2 in embodiment 1 are respectively immunized with conventional dosage 30-45kg, and the FMDV antibody is Negative pigs were 8. The prepared type A foot-and-mouth disease oil adjuvant vaccine (A / AKT-Ⅲ strain) and the type A foot-and-mouth disease compound water adjuvant inactivated vaccine were immunized with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com