Medicinal application of epimedin C in treatment on diabetic liver injury
A technology for diabetes and liver damage, applied in the field of preparation of drugs for the treatment of diabetic liver damage, can solve the problem of not fully considering the taste and bioavailability of drugs, and it is difficult to reveal the material basis of epimedium in the treatment of diabetes and its complications To achieve the effect of improving the oxidative stress state of the liver, alleviating the disorder of lipid metabolism, and increasing the level of HDL-C
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the preparation of epimedin C buccal tablet
[0042] A buccal tablet of Epimedin C for treating diabetic liver damage, its prescription is made of the following components by mass percentage: Epimedin C is 65%, arabinose is 9.8%, steviol glycoside is 0.1%, microcrystalline fiber 15% sodium cellulose, 9% hypromellose, 1% magnesium stearate, and 0.1% absolute ethanol.
[0043] The preparation method of Epimedin C buccal tablet is:
[0044] Step 1, weighing and pretreatment of raw materials: Weigh epimedin C, arabinose, steviol glycoside, microcrystalline cellulose, hypromellose and magnesium stearate respectively according to predetermined mass percentages, and pass through a 60-mesh sieve.
[0045] Step 2, making soft material: take Epimedin C, add arabinose and steviol glycoside, use absolute ethanol as wetting agent, stir well, add microcrystalline cellulose and hypromellose, stir well, and make soft material material.
[0046] Step 3, granulation: Put...
Embodiment 2
[0049] Embodiment 2: the preparation of epimedin C coated tablet
[0050] A kind of Epimedin C coated tablet for treating diabetic liver damage, its prescription is made of the following components by mass percentage: Epimedin C is 49.8%, microcrystalline cellulose is 15%, magnesium stearate is 1% , hypromellose 5%, steviol glycoside 0.2%, glycerin 1%, talc 1%, distilled water 27%.
[0051] The preparation method of Epimedin C coated tablet is:
[0052] Step 1, weighing and pretreatment of raw materials: Weigh the Epimedin C, microcrystalline cellulose, magnesium stearate and hypromellose respectively according to the predetermined mass percentage, and pass through a 60-mesh sieve.
[0053] Step 2, preparation of tablet cores: Take Epimedin C, add microcrystalline cellulose and hypromellose, mix well, use distilled water as a binder to make a soft material, and then put the wet granules into 60°C and blow dry Dry it in the box for about 1 hour, sprinkle magnesium stearate an...
Embodiment 3
[0056] Embodiment 3: the preparation of epimedin C hard capsule
[0057] An epimedin C capsule for treating diabetic liver damage, the prescription of which is made of the following components by mass percentage: epimedin C is 70%, and dietary fiber is 30%. In addition, several pharmaceutical gelatin hard capsule shells are required.
[0058] The preparation method of Epimedin C hard capsule is:
[0059] Step 1, raw material weighing and pretreatment: Weigh Epimedin C and dietary fiber respectively according to the predetermined mass percentage, and pass through a 60-mesh sieve.
[0060] Step 2, preparation of the filling material: mix the Epimedin C and the dietary fiber evenly.
[0061] Step 3, filling the capsule: adopt the free flow method to make the filling material enter the capsule shell, and then fit the capsule cap to obtain the Epimedin C hard capsule.
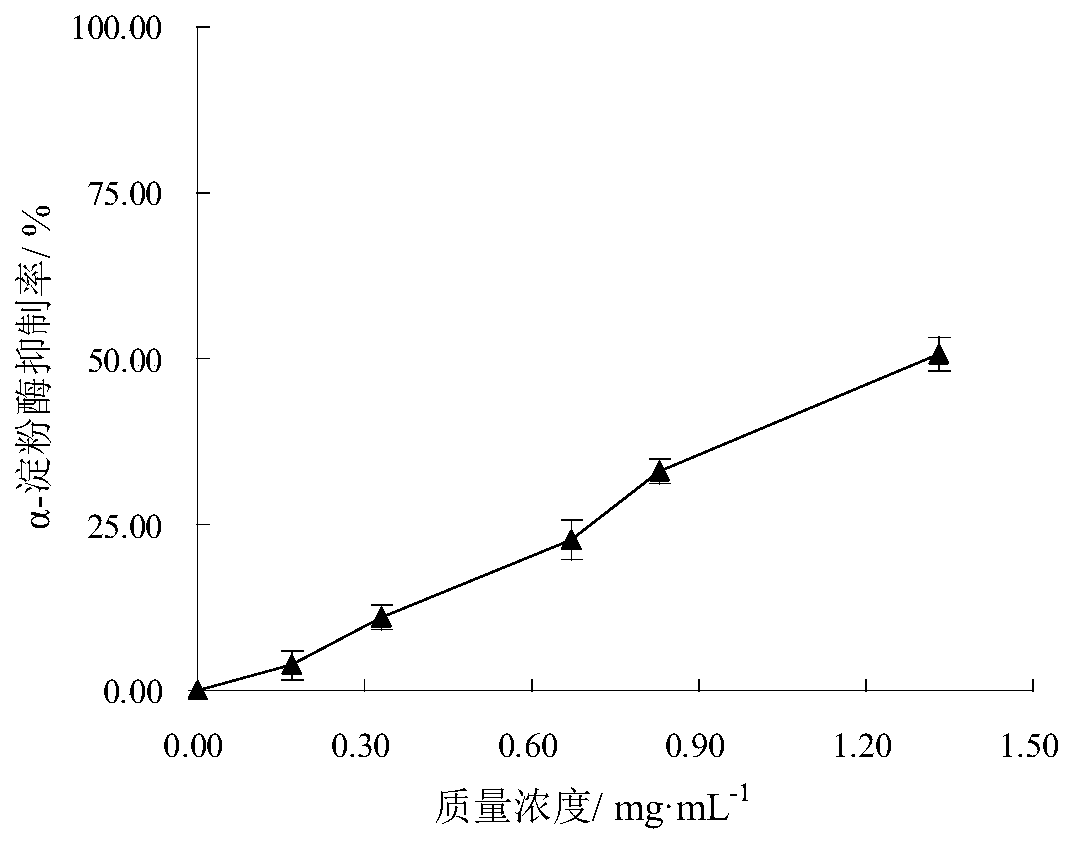
[0062] Further illustrate the therapeutic effect of Epimedin C of the present invention on diabetic liver dama...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com