All-organic conjugated polymer photocatalytic material and preparation method thereof
A conjugated polymer, photocatalytic material technology, applied in organic compound/hydride/coordination complex catalysts, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problem of poor photocatalytic performance, high cost, The preparation process is cumbersome and other problems, to achieve the effects of large specific surface area, high photoelectric effect, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Ultrasonic disperse 1 mL of pyrrole monomer into 100 mL (volume ratio: 0.01) of deionized water to form a dispersion, and place it in an ice bath for cooling. Dissolve 3.9g ferric chloride hexahydrate oxidizing agent in 40g water again (mass ratio is 0.1) to form uniform oxidizing agent solution, then it is added dropwise in the above-mentioned pyrrole monomer dispersion liquid with the speed of 50 drops / min, wait to stir After 0.5h, it was allowed to stand still for 12h to obtain a black polypyrrole organic novel photocatalyst. figure 1 Catalyst scanning electron microscope figure prepared for this embodiment, as figure 1 As shown, the polypyrrole prepared in this example is loose and porous, which is beneficial to the enrichment of pollutants and further to the photocatalytic reaction.
Embodiment 2
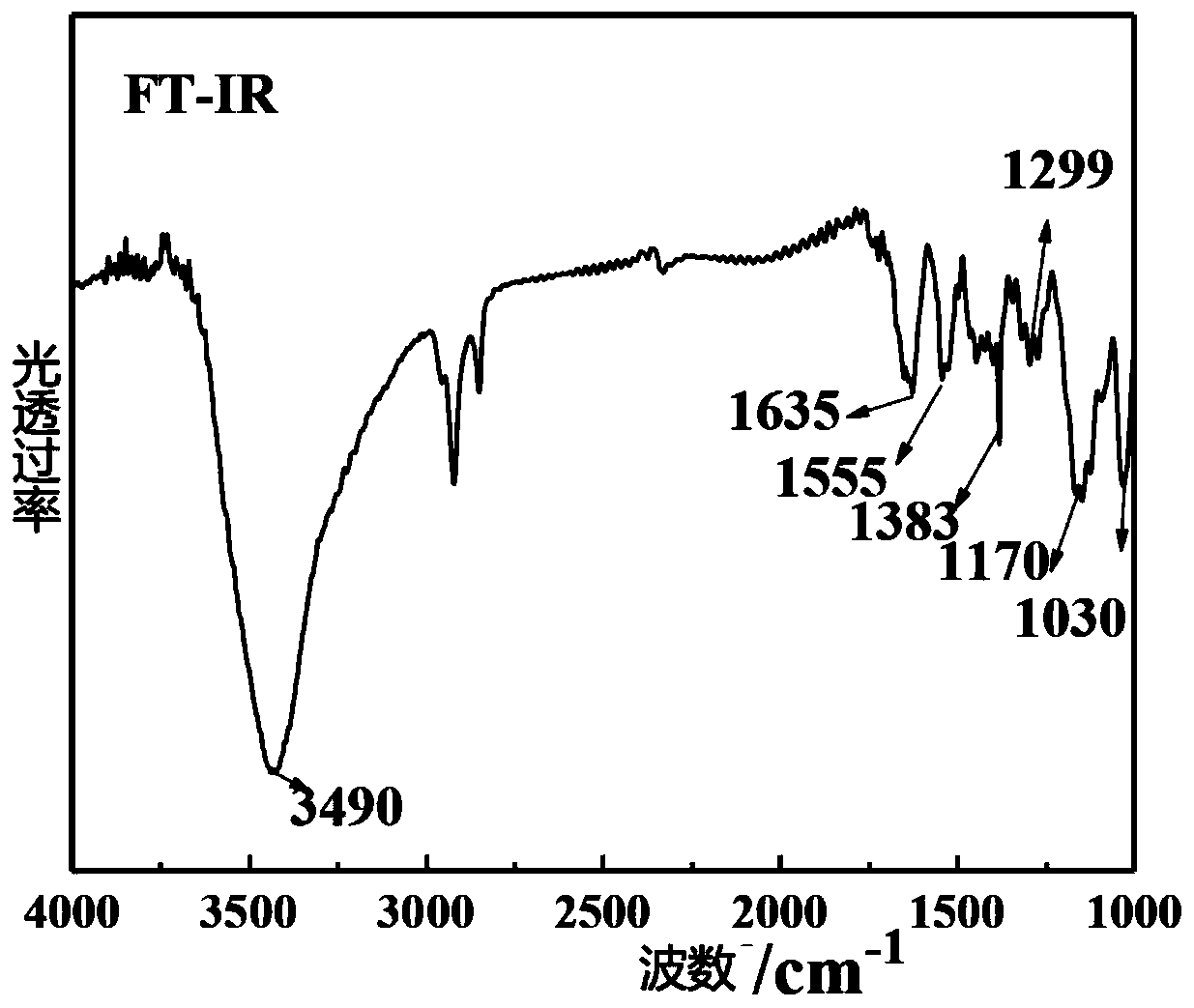
[0037] Implementation Example 2: Ultrasonic dispersion of 1.5mL of pyrrole monomer into 125mL (volume ratio: 0.12) of deionized water to form a dispersion liquid, placed in an ice bath for cooling treatment. Dissolve 4.136g of ammonium persulfate into 75g (mass ratio is 0.05) water to form a uniform oxidant solution, then it is added dropwise to the above-mentioned pyrrole monomer dispersion at a speed of 20 drops / min, and after stirring for 0.5h, Stand still for 24h to obtain a black polypyrrole organic novel photocatalyst. see figure 2 For the infrared spectrogram of the product that this embodiment obtains, as can be seen from the figure at 3490cm -1 N-H stretching vibration peak appeared at 1555cm -1 and 1383cm-1 They are the circular vibration and plane bending vibration of the typical C-H of polypyrrole. In addition, at 1299cm -1 The in-plane vibration peak of =C-H can be clearly observed. Therefore, the successful synthesis of polypyrrole photocatalytic materials c...
Embodiment 3
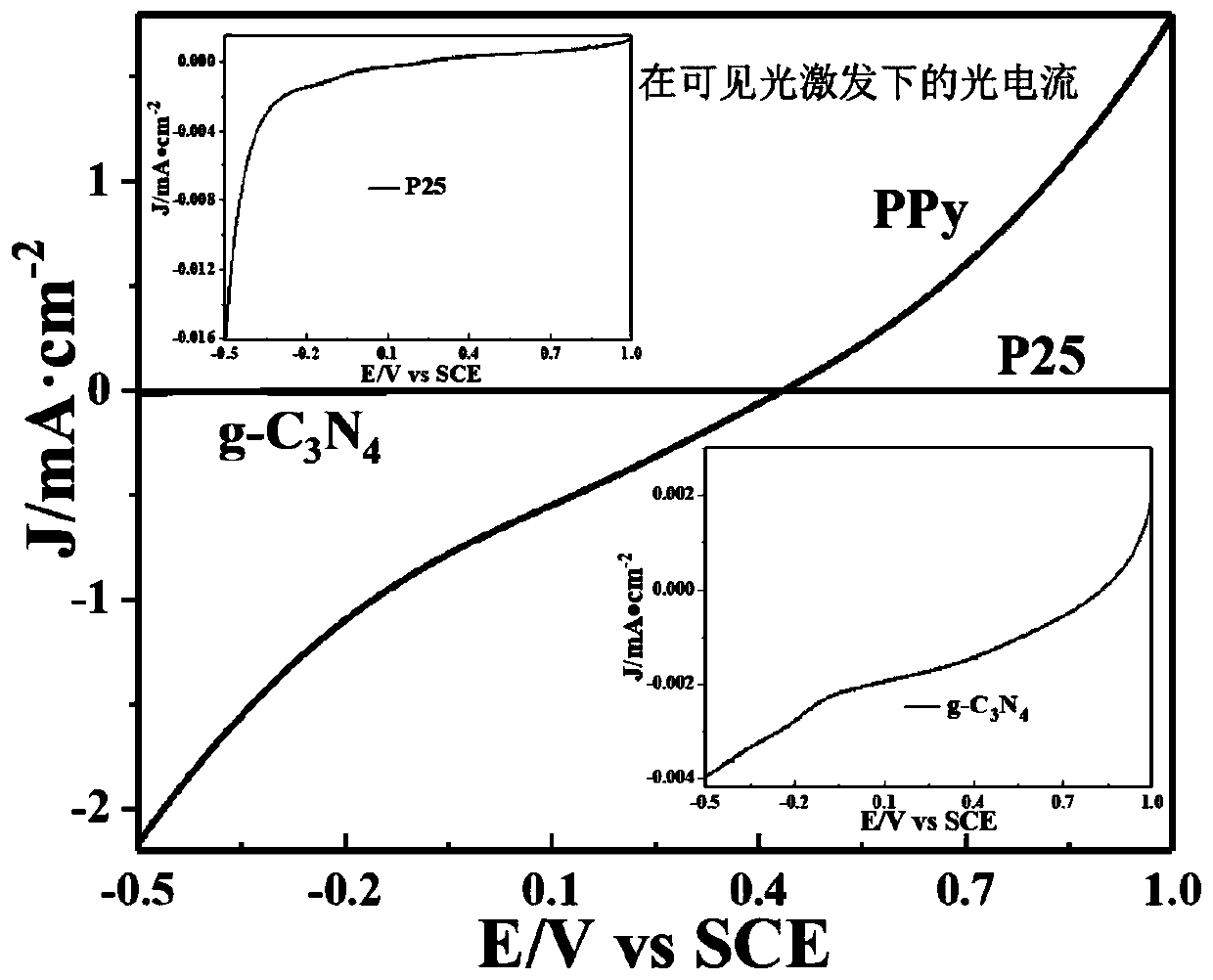
[0038] Implementation Example 3: 4.0 mL of pyrrole monomer was ultrasonically dispersed into 250 mL (volume ratio: 0.016) of deionized water to form a dispersion, and placed in an ice bath for cooling. Then 5.239g ferric chloride hexahydrate oxidant is dissolved in 125g (mass ratio is 0.05) water to form uniform oxidant solution, then it is added dropwise in the above-mentioned pyrrole monomer dispersion liquid with the speed of 50 drops / min, wait After stirring for 1 hour, it was allowed to stand still for 18 hours to obtain a black polypyrrole organic novel photocatalyst. Such as image 3 As shown, the polypyrrole prepared in this example has much higher photocurrent response intensity in the visible light region than P25 and g-C 3 N 4 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com