Helicobacter pylori HpaA subunit B cell epitope peptide and application
A technology of Helicobacter pylori and B cells, applied in the field of medicine and biology, can solve the problems of single anti-Hp treatment plan and increased drug resistance of anti-Hp treatment plan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0036] Example 1: Prediction of B-cell epitopes of Helicobacter pylori HpaA protein
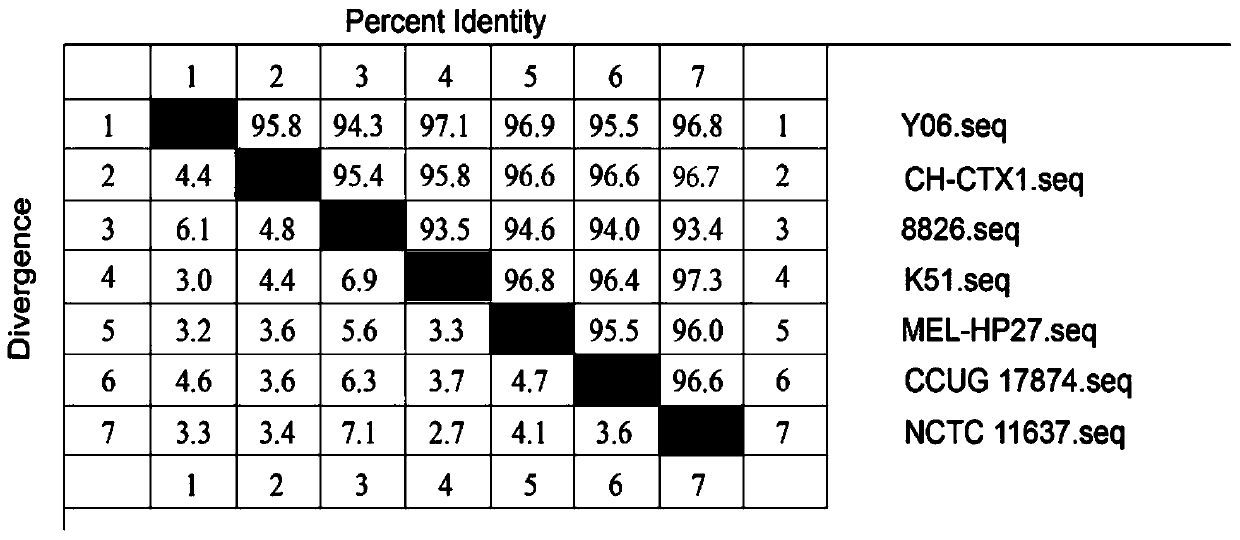
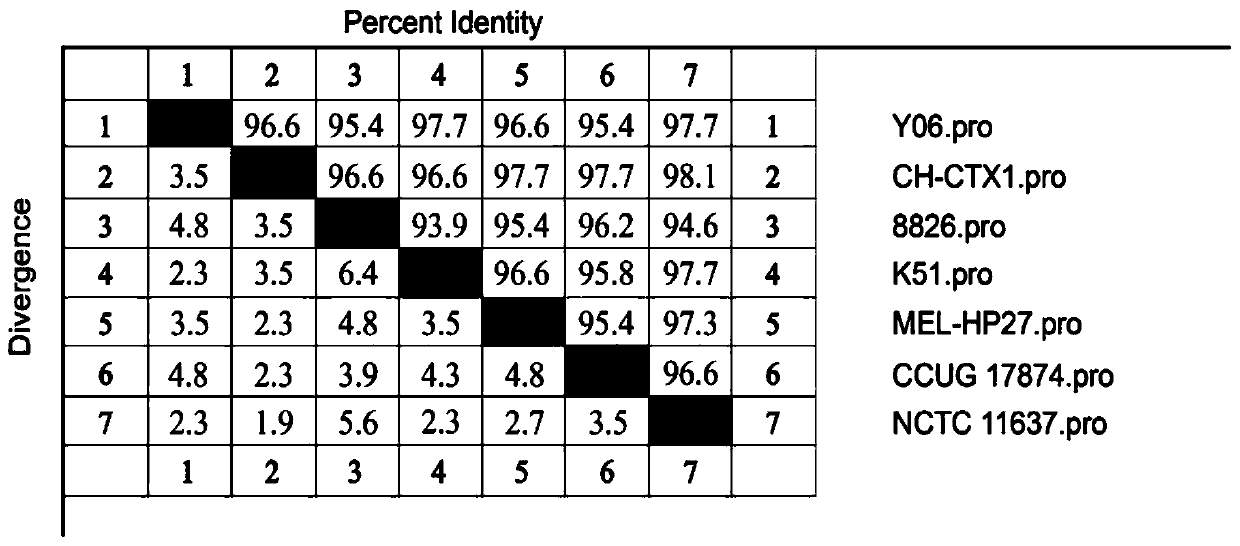
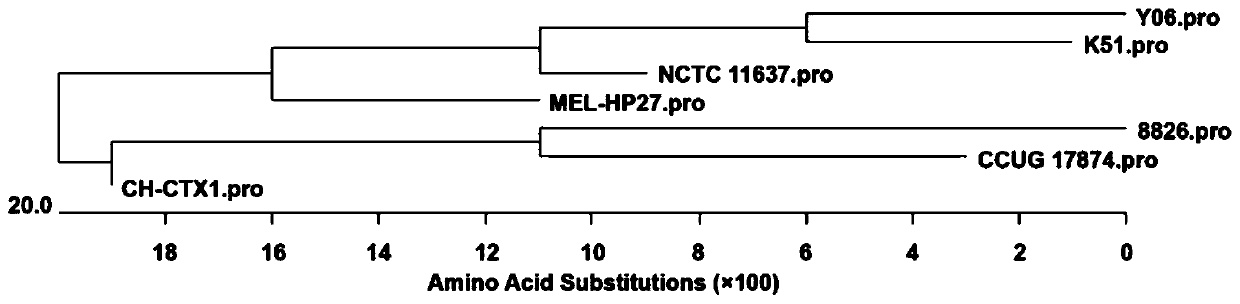
[0037] 1. Gene sequence analysis and strain selection Log in to the Genbank database (https: / / www.ncbi.nlm.nih.gov / genbank / ) to search for the nucleotide and amino acid sequences of Helicobacter pylori (Hp), using DNA Star Megalign software The nucleotide sequence and amino acid sequence of adhesin HpaA were compared. According to the results of phylogenetic tree analysis, a suitable strain was selected as the source strain for HpaA protein immune epitope analysis. There are 7 nucleotide sequences of adhesin HpaA registered in NCBI, the length of the open reading frame is 783bp, encoding 260 amino acids. Such as figure 1 with figure 2 As shown, the nucleotide and amino acid homology of seven Helicobacter pylori adhesin HpaA are 93.5%-97.3% and 93.9%-98.1%, respectively. The results of phylogenetic tree analysis showed that the adhesin HpaA sequence of the CH-CTX1 strain was closer to the...
example 2
[0045] Example 2: Screening of a synthetic multi-antigen peptide vaccine and its dominant epitope vaccine based on candidate epitopes
[0046] 1. Synthesis of B cell epitope multi-antigen peptide The synthesis of multi-antigen peptide was entrusted to Huaan Biochemical (Hangzhou) Co., Ltd. Based on the selected amino acid peptides and lysine as the core matrix, this example uses 8-branched peptides to design conformations and synthesize them on a polypeptide synthesizer by Fmoc solid-phase synthesis method. The polypeptides are purified by high-pressure liquid chromatography, and the purity is Reach more than 90%. For a schematic plan view of the preferred B cell epitope multi-antigen peptide, see Figure 13 . The 8-branch RPDPKRTIQKKSE constitutes MAP1, and the 8-branch GTDNSNDA constitutes MAP2.
[0047]2. The binding ability between MAP antigen peptide and Hp whole bacterial antibody was respectively coated with 100 μL / well of different MAP vaccines at a concentration of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com