Recombinant aspartase mutant, coding gene and application of recombinant aspartase mutant
An aspartase and mutant technology, applied in the application, genetic engineering, plant genetic improvement and other directions, can solve the problems of low yield, unfriendly environment, harsh reaction conditions, etc., and achieve high catalytic efficiency and environmental friendliness. , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1 produces wild-type aspartase LBS
[0029] The synthetic gene LBS was connected to the expression vector pET-28a(+), so that it could be expressed in Escherichia coli JM109(DE3).
[0030] The experimental operation process is as follows:
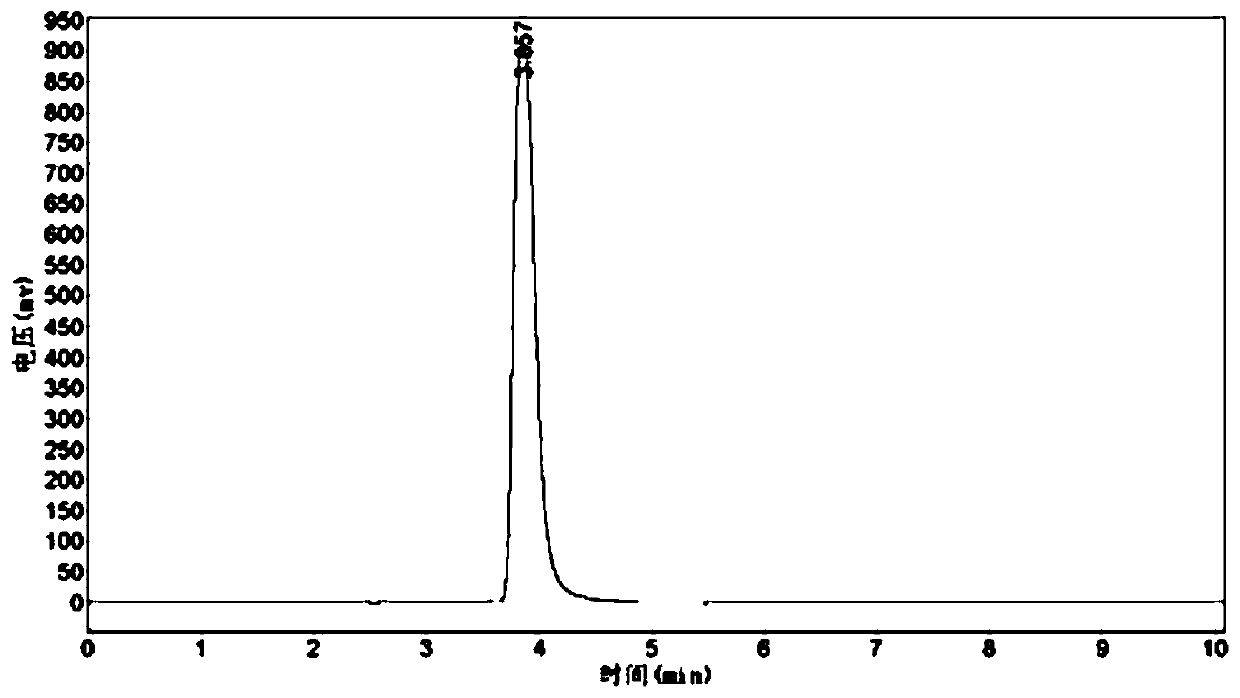
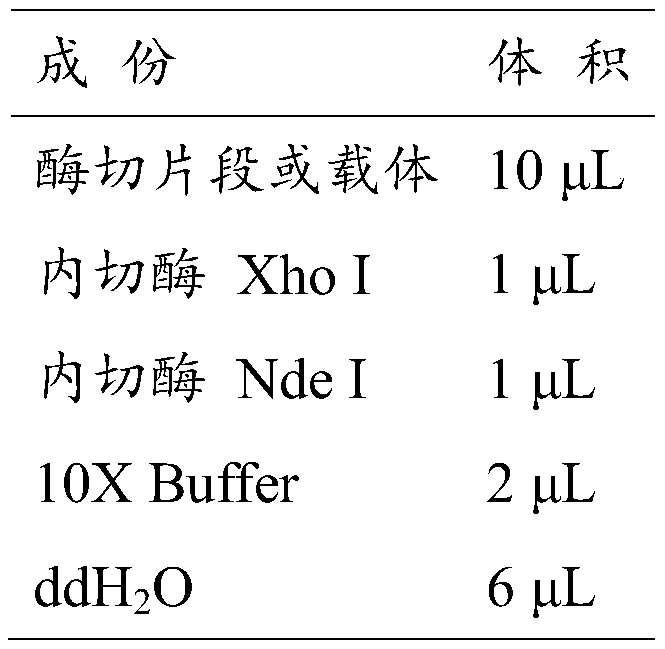
[0031] a) Use the same restriction enzymes to double-enzyme digest the nucleotide sequence SEQ ID No.4 encoding the amino acid sequence of SEQ ID No.1 and the expression vector pET-28a(+) to obtain sticky end fragments. After cutting for 100 min, mix with 10X Loading Buffer, pipette evenly, and then perform agarose gel electrophoresis. The 20 μL enzyme cutting system is shown in Table 1.
[0032] Table 1 Restriction enzyme digestion system
[0033]
[0034] b) Purify and recover the LBS gene fragment and pET-28a(+) vector fragment using the DNA gel recovery kit, see the instructions for the specific operation steps;
[0035] c) The recovered product was ligated into a circular plasmid using Ligation high (purchased f...
Embodiment 2
[0045] Embodiment 2 produces aspartase mutant LBS-2 and LBS-3
[0046] The synthetic gene LBS-2 was connected to the expression vector pET-28a(+), so that it could be expressed in Escherichia coli JM109(DE3).
[0047] The experimental operation process is as follows:
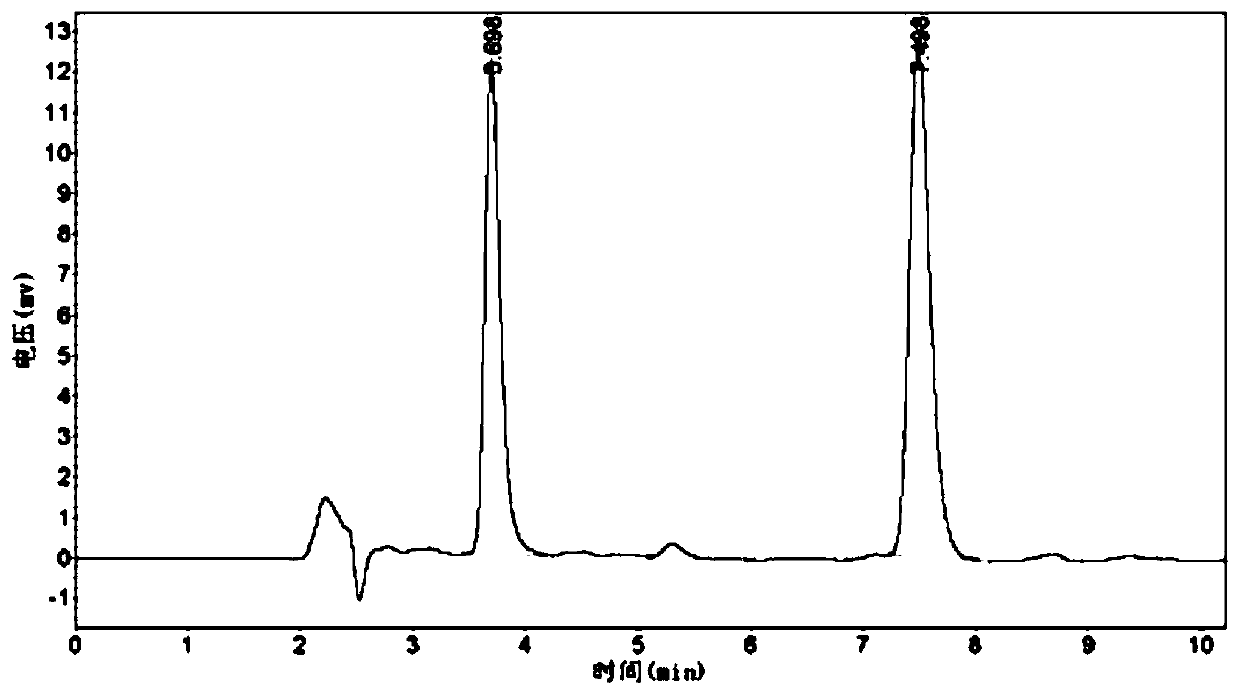
[0048] a) Use the same restriction enzymes to double-enzyme digest the nucleotide sequence SEQ ID No.5 encoding the amino acid sequence of SEQ ID No.2 and the expression vector pET-28a(+) to obtain sticky end fragments, and enzyme at 37°C After cutting for 100 min, mix with 10X Loading Buffer, pipette evenly, and run on agarose gel. See Table 3 for the 20 μL enzyme digestion system.
[0049] Table 3 Restriction enzyme digestion system
[0050]
[0051] b) Purify and recover the LBS-2 gene fragment and the pET-28a(+) vector fragment using a DNA gel recovery kit, see the instructions for the specific operation steps;
[0052] c) The recovered product was ligated into a circular plasmid using Ligation high (p...
Embodiment 3
[0063] Embodiment 3 wild aspartase LBS and recombinant aspartase mutant LBS-2 and LBS-3 protein purification
[0064] After equilibrating the protein Ni+ column with buffer A, add the crude enzyme solution prepared in Example 1, fully shake for 30 minutes, let go of the waste liquid, wash with buffer A three times, add buffer B and shake evenly for 20 minutes, and then collect the effluent , add excess ammonium sulfate to the effluent until it cannot be dissolved, centrifuge at 3000rpm for 10min, collect the precipitate, add 15% glycerol aqueous solution to dissolve the precipitate, and obtain a protein solution; The A280 absorption value of the solution was collected, and the part with an A280 absorption value greater than 0.1 was collected to obtain the purified wild-type aspartic acid LBS enzyme solution; buffer A: NaCl 140mmol / L, KCl 2.7mmol / L, NaCl 2 HPO 4 10mmol / L, KH 2 PO 4 1.8mmol / L, adjust the pH value to 8.0 with 5M NaOH, the solvent is water; buffer B: Na 2 HP...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com