Method for determining concentration of cefradine in blood plasma by liquid chromatography-tandem mass spectrometry
A technique of tandem mass spectrometry and liquid chromatography, applied in the field of drug analysis, can solve the problems such as the detection sensitivity of the determination method needs to be improved, the mobile phase of the determination method is complicated, the sample processing method is cumbersome, etc., to achieve excellent separation ability and avoid interference of endogenous substances. , The effect of sample pretreatment is simple
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: Determination of Cephradine in Plasma by Fast Liquid Chromatography-Tandem Mass Spectrometry
[0033] 1. Pretreatment of plasma samples:
[0034] Add 100 µL of plasma samples to a 96-well plate and add 50.0 µL of Tybipenem at a concentration of 250 ng . mL −1 300 µL of acetonitrile was added to the internal standard solution. Vortex for 5 min and centrifuge at 4500 rpm for 10 min at 4ºC. Pipette 50.0 µL of supernatant to a clean 96-well plate, add 100 µL of purified water and mix well. Take 2.00 µL of the supernatant for LC-MS / MS analysis.
[0035] 2. Preparation of standard series samples and quality control samples:
[0036] Weigh two parts of cephradine standard substance, dissolve with acetonitrile-water (1:1, v / v) to obtain a stock solution of cephradine with a concentration of 0.929mg / mL and 0.920mg / mL respectively, and one part is used for the standard series solution One for the preparation of quality control (QC) solution. Take the stock so...
Embodiment 2
[0042] Example 2: Methodological Validation
[0043] Carry out methodology verification to the assay method of embodiment 1, specifically as follows:
[0044] selectivity
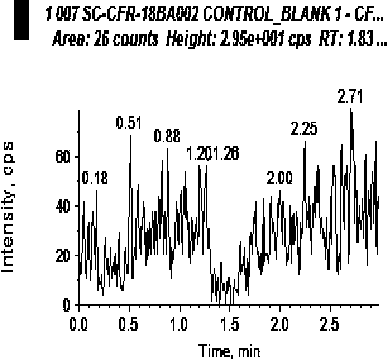
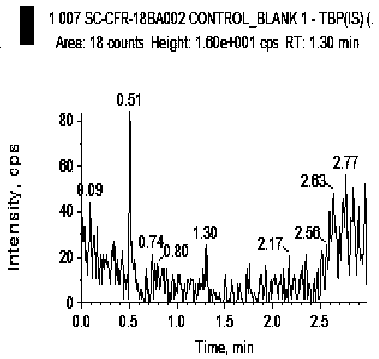
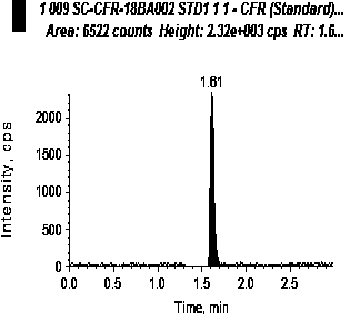
[0045] The blank human plasma and LLOQ samples were processed and analyzed respectively, and the corresponding chromatograms were obtained. The selectivity of the evaluation method.
[0046] The results showed that endogenous substances did not interfere with the determination of cephradine and tibipenem. For a typical chromatogram see figure 1 and figure 2 .
[0047] standard curve line
[0048] Take the theoretical concentration of cephradine as the abscissa (x), and the area ratio of cephradine and the internal standard tibipenem as the ordinate (y), and perform linear regression calculation (weight factor W=1 / x 2 ), the typical regression equation of cephradine is y=0.000764x+0.00248 (r=0.9996), and cephradine is obviously linear in the range of 60.00~30000ng / mL.
[0049] Accuracy and Precision...
Embodiment 3
[0057] Embodiment 3: clinical sample detection
[0058] The Human Bioequivalence Test of Cephradine Capsules was approved by the ethics committee and included 8 healthy subjects. The subjects took 250 mg / capsule of cephradine capsules test preparation (T) or reference preparation (R) orally on an empty stomach in two cycles respectively, blood samples were collected at 0 h (within 1 h before taking the medicine) and at different time points after taking the medicine, and separated to obtain After plasma, the concentration of cephradine in plasma was determined by an established method. A subject drug concentration - time curve see image 3 .
[0059] It can be seen from the foregoing examples that the present invention enhances the chromatographic retention of cephradine by optimizing the liquid chromatography conditions. Plasma samples were pretreated by protein precipitation. After the plasma sample is processed by protein precipitation, the extract can be directly injec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Collision gas | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com