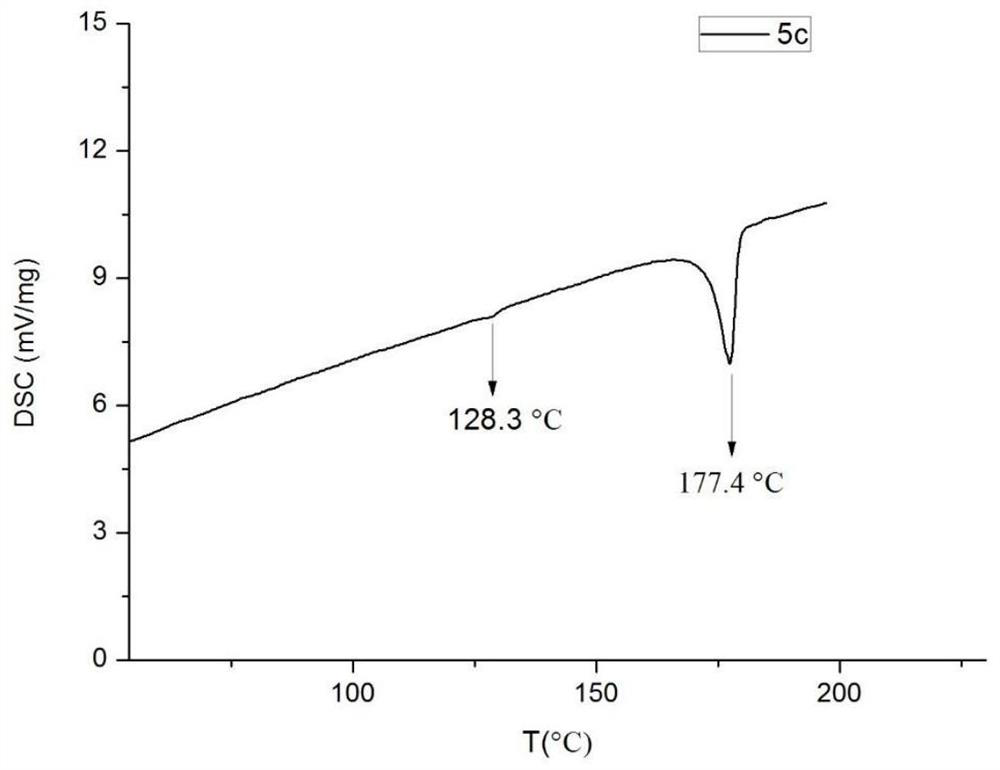
Difluoromethoxy liquid crystal compound containing benzoxazole heterocycle as well as synthesis method and application thereof
A technology of difluoromethoxyl and liquid crystal compounds, which is applied in chemical instruments and methods, liquid crystal materials, organic chemistry, etc., can solve problems such as the influence of the birefringence of liquid crystal molecules on the mesogenic phase type, and achieve a simple and easy synthesis method, Effect of High Refractive Index, Wide Mesophase Temperature Range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0079] Step S3 of the preparation method of the present invention has the advantage of simple reaction operation. Furthermore, the functionalization of transition metal-catalyzed carbon-hydrogen bonds conforms to the principles of green chemistry and atom economy. This method is used to construct CX(X=C, N, O, etc.) bonds have become a research hotspot in the field of organic chemistry. However, due to the unique physical properties of fluorine-containing groups, halogenated aromatic hydrocarbon substrates with difluoromethoxy groups as bridging bonds have not been reported, which makes their applications have certain limitations.
[0080] Therefore, the present invention uses Pd(AcO)2 as the catalyst, NiXantphos as the ligand, and NatOBu as the base to react in THF or DME at 65°C for 12h, which is the first realization of a halogen with difluoromethoxy as a bridge. Arylation reaction of substituted aromatic hydrocarbon substrates. The reaction operation is simple, and ordinary ...
Embodiment 1
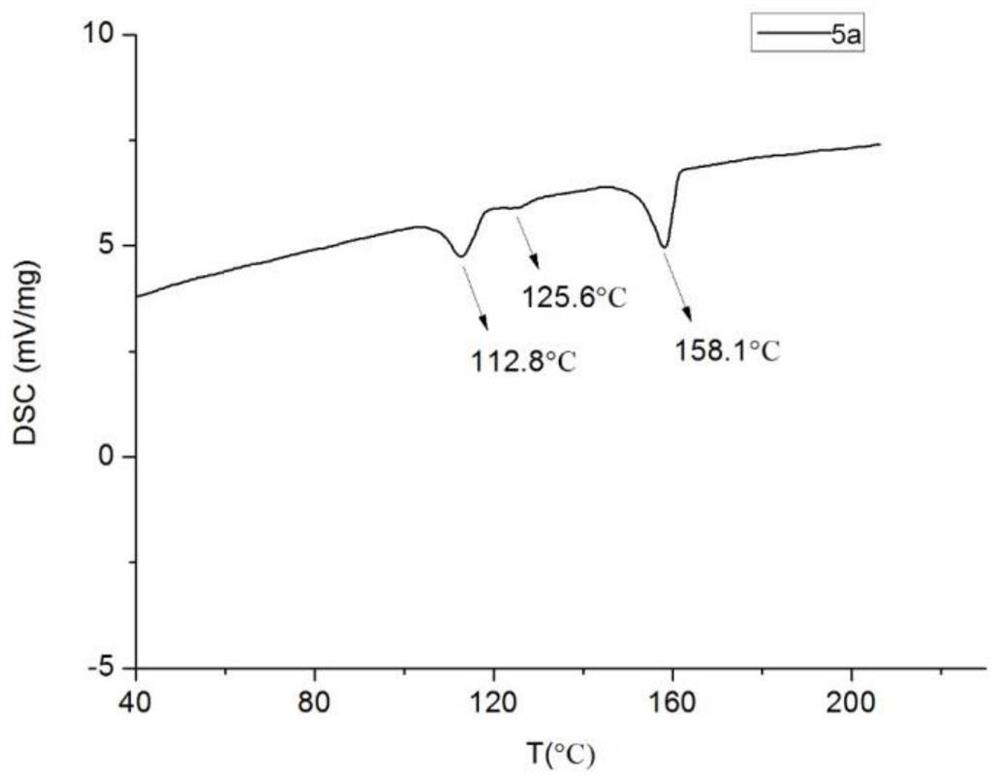
[0086] The synthesis of 2-[4-(trans-4-(trans-4-n-propylcyclohexyl)cyclohexyldifluoromethoxy)phenyl]benzoxazole (5a) includes the following steps:
[0087] (1) Synthesis of 1-(4-(4-(trans-4-n-propylcyclohexyl)cyclohexyldifluoromethoxy)bromobenzene (3CCBr-1)
[0088] Trans-4-(trans-4-n-propylcyclohexyl) cyclohexyl carboxylic acid 3CCA (6.2200g, 0.0240mol) (structure is ), toluene (12.0 mL), isooctane (12.0 mL) and 1,3-propanedithiol (2.4 mL, 0.0240 mol) were added to a dry 250 mL three-neck round bottom flask. The mixture was stirred at 50°C for 1 hour, and trifluoromethanesulfonic acid (2.0 mL, 0.0240 mol) was slowly added dropwise (0.5 h) to the reaction solution using a dropping funnel. Heat to 102-105°C, reflux and separate water for about 4 hours. After the water was separated, the solvent was distilled off under reduced pressure, and 20.0 mL of the dichloromethane solution after dewatering was added to obtain the disulfide salt (3CCB) in dichloromethane solution. The entire r...
Embodiment 2
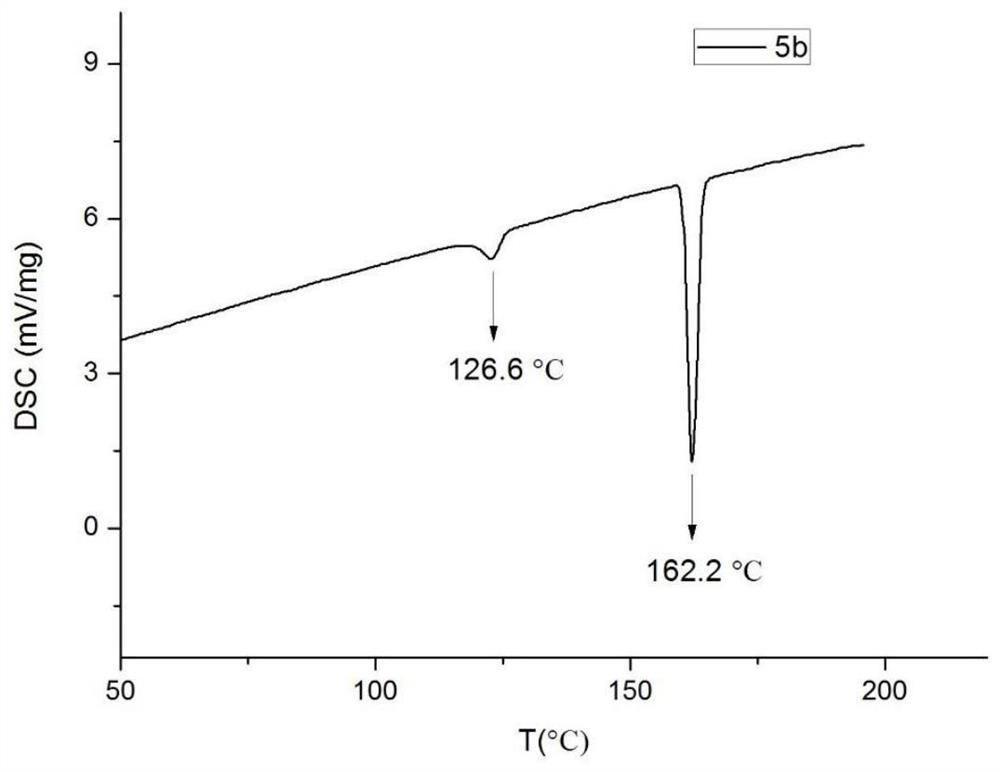
[0097] The synthesis of 2-[4-(trans-4-(trans-4-n-propylcyclohexyl)cyclohexyldifluoromethoxy)phenyl]-5-methylbenzoxazole (5b) includes the following steps :
[0098] (1) Synthesis of 1-(4-(4-(trans-4-n-propylcyclohexyl)cyclohexyldifluoromethoxy)bromobenzene (3CCBr-1)
[0099] The same as in Example 1.
[0100] (2) Synthesis of 2-[4-(trans-4-(trans-4-n-propylcyclohexyl)cyclohexyldifluoromethoxy)phenyl]-5-methylbenzoxazole (5b)
[0101] The product in (1) 3CCBr-1 (0.0950g, 0.0002mol), 5-methylbenzoxazole (0.0319, 0.00024mol), Pd(OAc) 2 (0.0025g, 0.005%mol), NiXantphos (0.0110g, 0.01%mol) and NatOBu (0.0480g, 0.0005mol) were dissolved in THF, heated to 65°C, and reacted for 12h. After cooling, it was filtered, extracted with ethyl acetate (30.0 mL) three times, washed, and dried over anhydrous sodium sulfate. Finally, column chromatography was performed with petroleum ether: ethyl acetate (V:V=50:1) to obtain a white solid with a yield of 78%.
[0102] The structural characterization dat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com