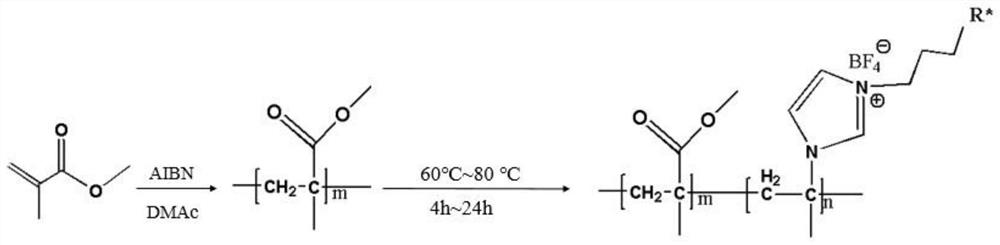
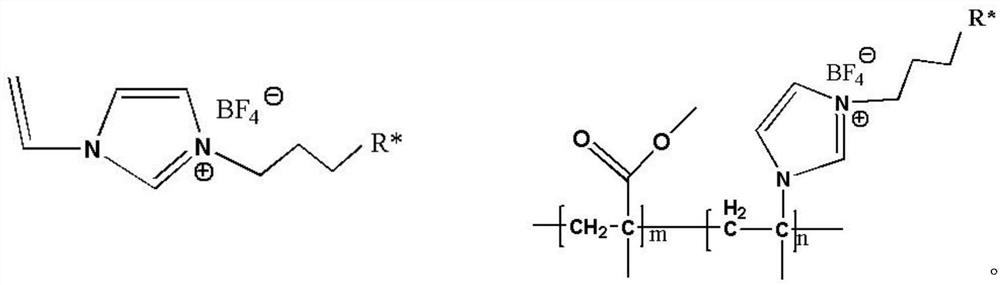
Synthesis of imidazole functionalized ionic liquid copolymer and preparation method of alloy ultrafiltration membrane
A technology of ionic liquids and copolymers, which is applied in the field of membrane separation, can solve the problems of unstable synthesis of ionic liquid copolymers, the performance of easily polluted small molecule ionic liquids, and poor hydrophilicity of alloy ultrafiltration membranes, and achieve free radical polymerization. Control, anti-pollution effect is remarkable, the effect of a wide range of applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1) Synthesis of amphiphilic PMMA-b-PIL-R*
[0026] Dissolve 4.005g of MMA in dimethylacetamide solvent and place in a flask, stir magnetically, feed nitrogen into the reaction system, remove oxygen, and raise the temperature of the reaction bath to 60°C, add 0.049g of azobisisobutyl Nitrile initiator, under the protection of nitrogen, react for 2h. Weigh 5.081 g of 1-hydroxybutyl-3-vinylimidazolium tetrafluoroborate into the reaction system, and continue the reaction for 4 h under the protection of nitrogen. After the reaction, the resulting copolymer solution was immediately put into a water cooler containing an ice-water solution to cool to terminate the reaction. Then the copolymer solution is poured into ethanol for precipitation, and the product is filtered through deionized water and washed 3 times, then put into a freeze dryer and freeze-dried for 12h to obtain a white copolymer (PMMA-b-PIL-R 1 *), PMMA-b-PIL-R 1 *The value of m is 50 and the value of n is 60....
Embodiment 2
[0040] 1) Synthesis of amphiphilic PMMA-b-PIL-R*
[0041] Dissolve 10.012g MMA in dimethylacetamide solvent and place in a flask, stir magnetically, feed nitrogen into the reaction system, remove oxygen, raise the temperature of the reaction bath to 70°C, add 0.049g azobisisobutyl Nitrile initiator, reacted for 3h under nitrogen protection. Weigh 6.361 g of 1-sulfonic acid butyl-3-vinylimidazolium tetrafluoroborate and add it into the reaction system, and continue the reaction for 12 h under the protection of nitrogen. After the reaction, the resulting copolymer solution was immediately put into a water cooler containing an ice-water solution to cool to terminate the reaction. Then the copolymer solution is poured into absolute ethanol for precipitation, and the product is filtered through deionized water and cleaned 3 times, then put into a freeze dryer and freeze-dried for 10h to obtain a white copolymer (PMMA-b-PIL-R 2 *), PMMA-b-PIL-R 2 *The value of m is 70 and the val...
Embodiment 3
[0045] 1) Synthesis of amphiphilic PMMA-b-PIL-R*
[0046] Dissolve 6.072g of MMA in dimethylacetamide solvent and place in a flask, stir magnetically, feed nitrogen into the reaction system, remove oxygen, and raise the temperature of the reaction bath to 80°C, add 0.049g of azobisisobutyl Nitrile initiator, reacted under nitrogen protection for 4h. Weigh 5.641 g of 1-carboxybutyl-3-vinylimidazolium tetrafluoroborate monomer into the reaction system, and continue the reaction for 24 hours under the protection of nitrogen. After the reaction, the resulting copolymer solution was immediately put into a water cooler containing an ice-water solution to cool to terminate the reaction. Then the copolymer solution is poured into ethanol for precipitation, and the product is filtered through deionized water and washed 3 times, then put into a freeze dryer and freeze-dried for 15h to obtain a white copolymer (PMMA-b-PIL-R 3 *), PMMA-b-PIL-R 3 *The value of m is 100 and the value of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water contact angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com