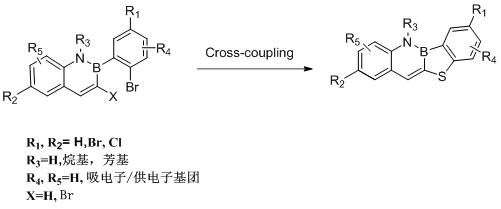
Synthesis method of benzoboron-nitrogen naphthalene thiophene derivative
A benzoborazine-naphthylthiophene and synthetic method technology, applied in chemical instruments and methods, compounds containing elements of group 3/13 of the periodic table, organic chemistry, etc., to achieve the effect of wide range of raw materials, small impact and simple reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Synthesis of benzoborazine-naphthylthiophene derivatives:
[0017] Take 15 mL of dry Schlenk tube, evacuate and change argon three times, under the protection of argon, add bisbromoborazine (362 mg, 1 mmol), Na 2 S (156 mg, 2 mmol), CuI (19 mg, 10 mol %), 4,4'-di-tert-butyl-2,2'-dipyridine (54 mg, 20 mol %) Na 2 CO 3 (160 mg, 1.5 mmol), introduced into anhydrous and oxygen-free solvent DMF (5mL). Under the protection of argon, react at 120°C for about 8 h. After the reaction is completed, after the temperature of the system drops to room temperature, extract with dichloromethane (4*10 mL) and water (50 mL), combine the organic phases, and dry them with anhydrous sodium sulfate. , the solvent was removed under reduced pressure, and column chromatography (developing solvent: n-hexane: dichloromethane = 5:1) gave 160 mg of a yellow-green solid with a yield of 68%.
[0018] The 1H NMR spectrum, 13C, and 11B NMR spectrum were measured with a 400M superconducting nuclea...
Embodiment 2
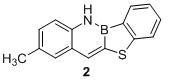
[0022] Synthesis of benzoborazine-naphthylthiophene derivative compound 2:
[0023]
[0024] Take 15 mL of dry Schlenk tube, vacuumize and change argon three times, under the protection of argon, add monobromoborazin (297 mg, 1 mmol), Na 2 S (156 mg, 2 mmol), CuI (19 mg, 10 mol %), 4,4'-di-tert-butyl-2,2'-dipyridine (54 mg, 20 mol %), Na 2 CO 3 (160 mg, 1.5 mmol), introduced into anhydrous and oxygen-free solvent tetrahydrofuran (5 mL). Under the protection of argon, react at 140°C for about 6 h. After the reaction is completed, after the temperature of the system drops to room temperature, extract with dichloromethane (4*10 mL) and water (50 mL), combine the organic phases, and dry them with anhydrous sodium sulfate. , the solvent was removed under reduced pressure, and column chromatography (developing solvent: n-hexane: dichloromethane = 5:1) gave 181 mg of a yellow-green solid with a yield of 72%.
[0025] The nuclear magnetic analysis data of this compound are as f...
Embodiment 3
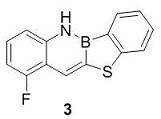
[0028] Synthesis of benzoborazine-naphthylthiophene derivative compound 3:
[0029]
[0030] Take 15 mL of dry Schlenk tube, vacuumize and change argon three times, under the protection of argon, add monobromoborazin (301 mg, 1 mmol), Na 2 S (156 mg, 2 mmol), CuI (19 mg, 10 mol %), 2,2'-bipyridine (32 mg, 20 mol %), Na 2 CO 3 (160 mg, 1.5 mmol), introduced into anhydrous and oxygen-free solvent tetrahydrofuran (5 mL). Under the protection of argon, react at 100°C for about 18 h. After the reaction is completed, after the temperature of the system drops to room temperature, extract with dichloromethane (4*10 mL) and water (50 mL), combine the organic phases, and dry over anhydrous sodium sulfate. The solvent was removed under reduced pressure and separated by column chromatography (developing solvent: n-hexane: dichloromethane = 5:1) to obtain 178 mg of a yellow-green solid with a yield of 70%.
[0031] The nuclear magnetic analysis data of this compound are as follows: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com