Antibody binding to human LAG-3 as well as preparation method and application of antibody
A technology of LAG-3 and antibody, which is applied in botany equipment and methods, biochemical equipment and methods, antibodies, etc., to achieve good clinical application prospects and enhance the effect of biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Example 1. Preparation of human LAG-3 protein and anti-LAG-3 positive control antibody
[0078] The amino acid sequence of the extracellular segment of human LAG-3 was obtained from https: / / www.uniprot.org / uniprot / P18627 , the amino acid sequence is shown in SEQ ID NO: 41, codon optimization was performed by Sangon Bioengineering (Shanghai) Co., Ltd. and the gene was synthesized, and then the synthesized gene fragment was cloned into the pUC57 vector. Primers were designed to amplify the LAG-3 extracellular domain (Extracellular Domain, ECD) coding region (SEQ ID NO: 41) and the Fc segment coding region (SEQ ID NO: 51) of human immunoglobulin IgG1 by PCR (Polymerase Chain Reaction) method ); recovering the above-mentioned amplified fragments, splicing the above-mentioned fragments together by recombinant PCR, introducing the coding region of the signal peptide (MGVKVLFALICIAVAEA, SEQ ID NO: 42) at the same time, and introducing corresponding enzyme cutting sites at bot...
Embodiment 2
[0080] Example 2. Preparation and screening of mice immunized with human LAG-3-ECD-hFc as antigen and hybridoma
[0081] After diluting the LAG-3-ECD-hFc prepared in Example 1 to an appropriate concentration with physiological saline, mixed with an equal volume of Freund's complete adjuvant (purchased from Sigma), after phacoemulsification, the 4-5-week-old Balb / c Mice (purchased from Shanghai Lingchang Biotechnology Co., Ltd., animal production license number: SCXK (Shanghai) 2013-0018) were injected subcutaneously at multiple points; three weeks later, LAG-3-ECD-hFc was diluted with normal saline and mixed with An equal volume of Freund's incomplete adjuvant (purchased from Sigma) was mixed, and after phacoemulsification was complete, the mice were injected subcutaneously at multiple points; this step was repeated two weeks later; all mice were harvested on the seventh day after the third immunization. Serum was separated from a small amount of blood samples, and the serum t...
Embodiment 3
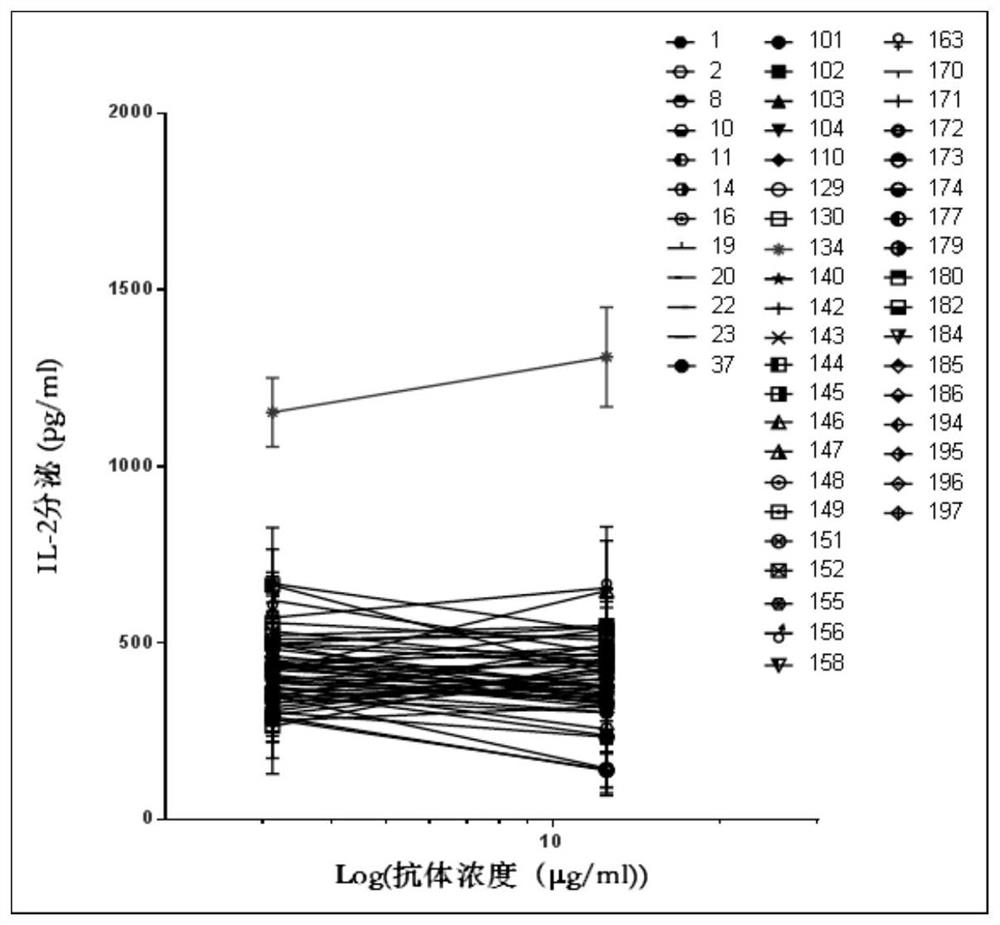
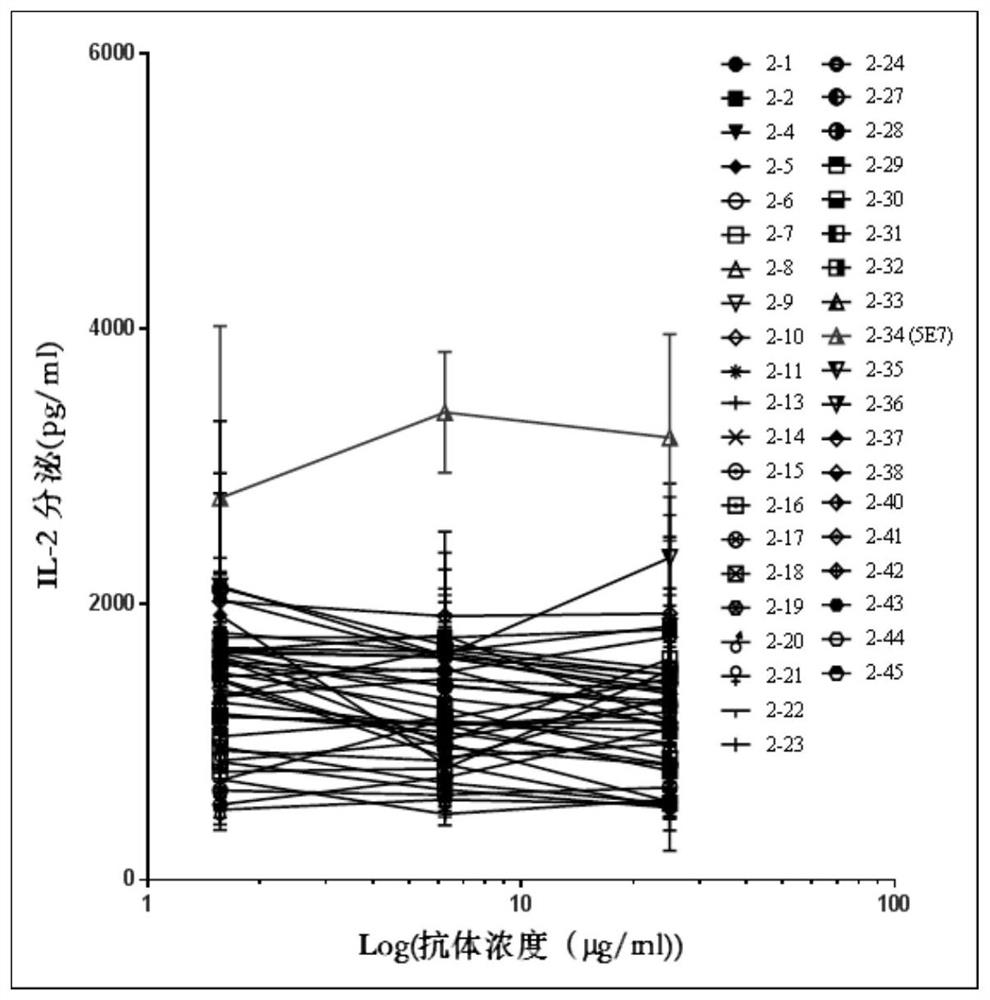
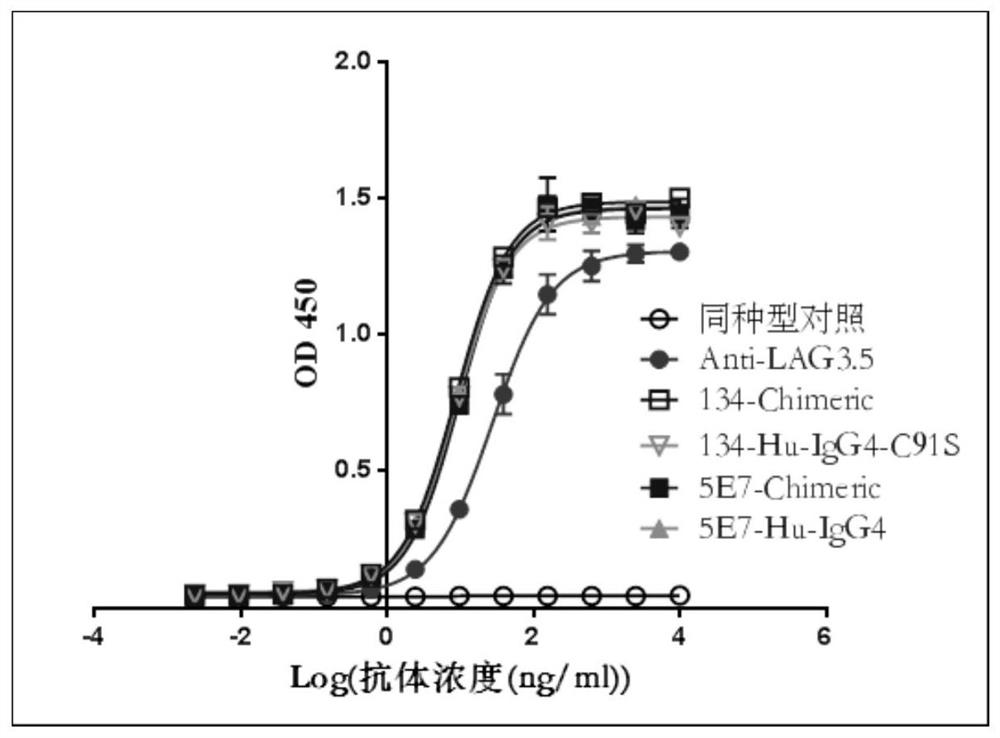
[0086] Example 3. Effect of mouse-derived anti-human LAG-3 antibody on mixed lymphocyte reaction
[0087] Here, the mixed lymphocyte reaction (Mixed Lymphocyte Reaction, MLR) was used to evaluate the activity of the murine anti-human LAG-3 antibody, and Anti-LAG3.5 was added as a positive control.
[0088] The MLR experimental method is described as follows: use Histopaque (purchased from Sigma) to isolate peripheral blood mononuclear cells (Peripheral Blood Mononuclear Cell, abbreviated as PBMC) from human blood, and isolate the mononuclear cell subpopulation in the PBMC by the adherence method, Then IL-4 and GM-CSF were combined to induce monocytes to differentiate into induced dendritic cells (p38MAPK-inhibited dendritic cells induce superior antitumour immune responses and overcome regulatory T-cell-mediated immunosuppression. NatCommun.2014Jun 24; 5:4229 .doi:10.1038 / ncomms5229.); Seven days later, the above-mentioned induced dendritic cells were collected by digestion; P...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com