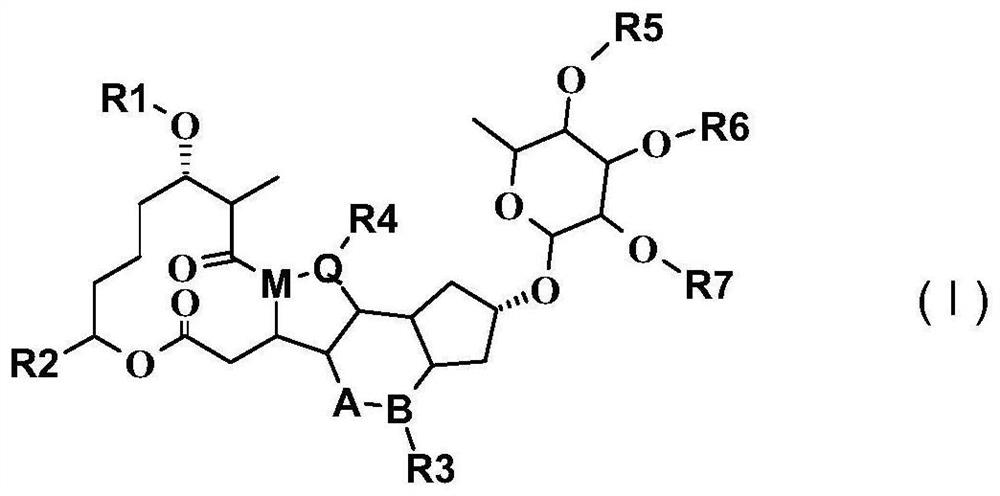
Spinosyn derivative as argininosuccinate synthetase activator and application of spinosyn derivative
A technology of argininosuccinic acid and argininosuccinic acid, which is applied in the field of medicine and achieves remarkable curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Target identification and confirmation experiments
[0067] The specific test steps are as follows:
[0068] 1) Synthesis of biotin-based spinosyn probe spinosyn-biotin complex Xn-03-17A.
[0069] (1) Synthesis of N-(3-bromohexyl) spinosyn: add 1 g (1.39mmoL) of spinosyn B, 20ml of acetonitrile and 385mg (1.39mmol) of potassium carbonate to a 100mL single-necked round bottom flask, and finally add 1,6-Dibromohexane 2.14 mL (13.9mmoL), stirred at 45°C for 48h. The resulting solid was removed by suction filtration, the solvent was removed, extracted with 50 mL of water and EA (3×60 mL), the combined organic phases were dried over anhydrous sodium sulfate. After removal of the solvent, 840 mg of white solid was obtained through silica gel column layer V (ethyl acetate): V (petroleum ether) = 4:1, yield 73%. m.p.95~98℃; HRMS calcd. for C 46 h 74 BrNO 10 880.4574, found 880.4602.
[0070] (2) Synthesis of spinosyn-hexyl-biotin (Xn-03-17A): Add DMF1.5 mL, compound 510...
Embodiment 2
[0075] Effects of Drugs on Protein ASS1 Enzyme Activity
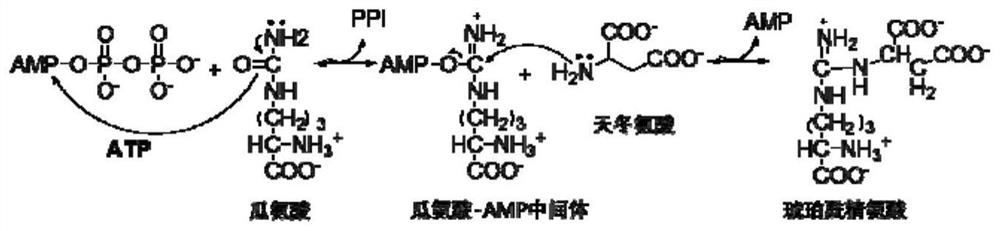
[0076] The principle of ASS1 activity detection is: ASS1 catalyzes the reaction of citrulline and aspartic acid to generate argininosuccinic acid, which consumes ATP to generate pyrophosphate (PPi), and the generated PPi is produced under the catalysis of pyrophosphatase Phosphoric acid, phosphoric acid reacts with ammonium molybdate to generate phosphomolybdenum heteropolyacid, and under the reduction of vitamin C, it generates blue phosphomolybdenum blue, which has a characteristic absorption peak at OD660.
[0077] Enzyme activity calculation formula:
[0078] Enzyme activity is: [(drug+ASS1)OD 660 -NCOD 660 ] / (ASS1OD 660 -NCOD 660 )×100%.
[0079] (drug+ASS1)OD 660 : Solution absorbance (wavelength 660nm) when adding medicine and ASS1; ASS1OD 660 :
[0080] Solution absorbance when adding ASS1 Solution absorbance (wavelength 660nm); NCOD 660 : Blank reference solution absorbance (wavelength 660nm).
[0081...
Embodiment 3
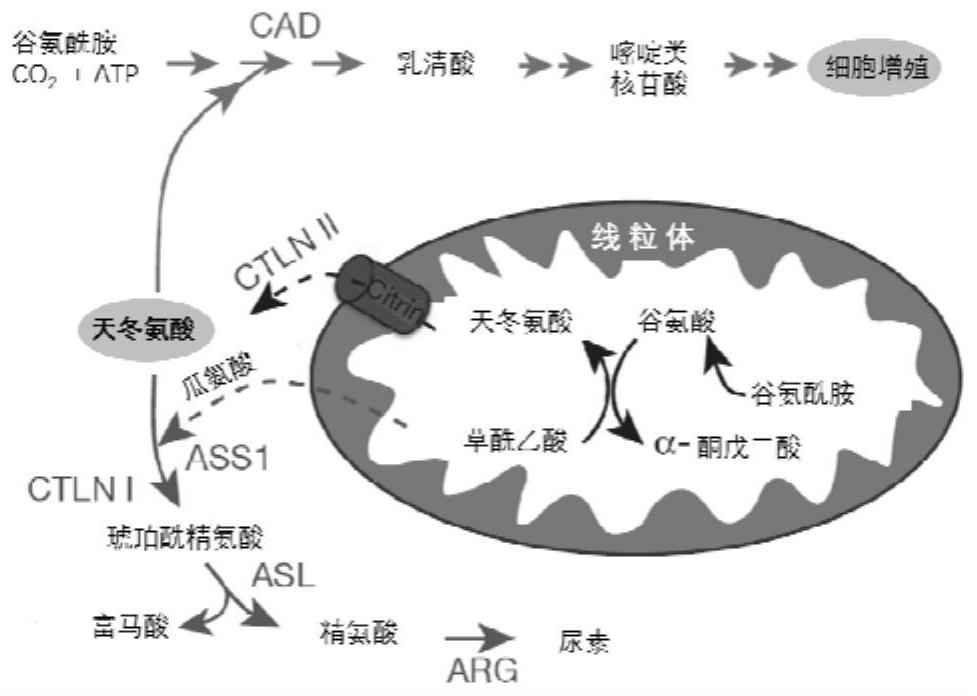
[0098] Drugs against proteins ASS1 and ASS1 G362V Effect of enzyme activity
[0099] In order to detect SPA and its derivatives LM-2I on the protein ASS1 and ASS1 G362V Enzyme activity, we constructed the ASS1 pET28a plasmid through homologous recombination technology, and on this basis, used gene site-directed mutagenesis technology to construct ASS1 G362V pET28a plasmid, the constructed plasmid was transferred into BL21(DE3) for prokaryotic expression and protein purification of ASS1 and ASS1 G362V .
[0100] The specific steps are:
[0101] 3.1 ASS1 and ASS1 G362V amino acid sequence
[0102] The amino acid sequence of ASS1 is:
[0103]MGSSHHHHHHSSGLVPRGSHMASMTGGQQMGRGSEFMSSKGSVVLAYSGGLDTSCIL VWLKEQGYDVIAYLANIGQKEDFEEARKKALKLGAKKVFIEDVSREFVEEFIWPAIQSSALY EDRYLLGTSLARPCIARKQVEIAQREGAKYVSHGATGKGNDQVRFELSCYSLAPQIKVIAP WRMPEFYNRFKGRNDLMEYAKQHGIPIPVTPKNPWSMDENLMHISYEAGILENPKNQAPP GLYTKTQDPAKAPNTPDILEIEFKKGVPVKVTNVKDGTTHQTSLELFMYLNEVAGKHGVGR IDIVENRFIGMKSRGIYETPAGTILYHAHLDI...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com