Catalyst for synthesizing salicylic acid through phenol carboxylation reaction and preparation method
A technology of phenol carboxyl group and catalyst, which is applied in the field of catalyst and preparation of phenol carboxylation reaction to synthesize salicylic acid, which can solve the problems of low reaction activity, harsh reaction conditions and high reaction efficiency, and achieve strong solubility, simple recycling and catalytic highly active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of [Bmim]Br / AlBr 3 : Step 1 {[Bmim]Br}: Weigh 64.12g (0.78mol) of 1-methylimidazole into a 500ml three-necked flask, and stir rapidly at a constant temperature of 70°C. Slowly add 117.87 g (0.858 mol) of n-bromobutane dropwise into the three-necked flask with a constant pressure dropping funnel for 1 h. After the dropwise addition, the mixture was refluxed at 70° C. for 10 h. After the reaction was completed, cool to room temperature to obtain the crude product [Bmim]Br, which was transferred to a separatory funnel, washed (3 times) with 30ml ethyl acetate, and then the product was rotary evaporated at 60°C for 30min to remove residual ethyl acetate. ester. The remaining product was transferred to a beaker and placed in a vacuum drying oven at a temperature of 65°C, and dried to constant weight to obtain the finished product of 1-methyl-3-n-butylimidazole bromide ([Bmim]Br). Step 2 {[Bmim]Br / AlBr 3}: Weigh 50g of prepared [bmim]Br into a 250ml single-nec...
Embodiment 2
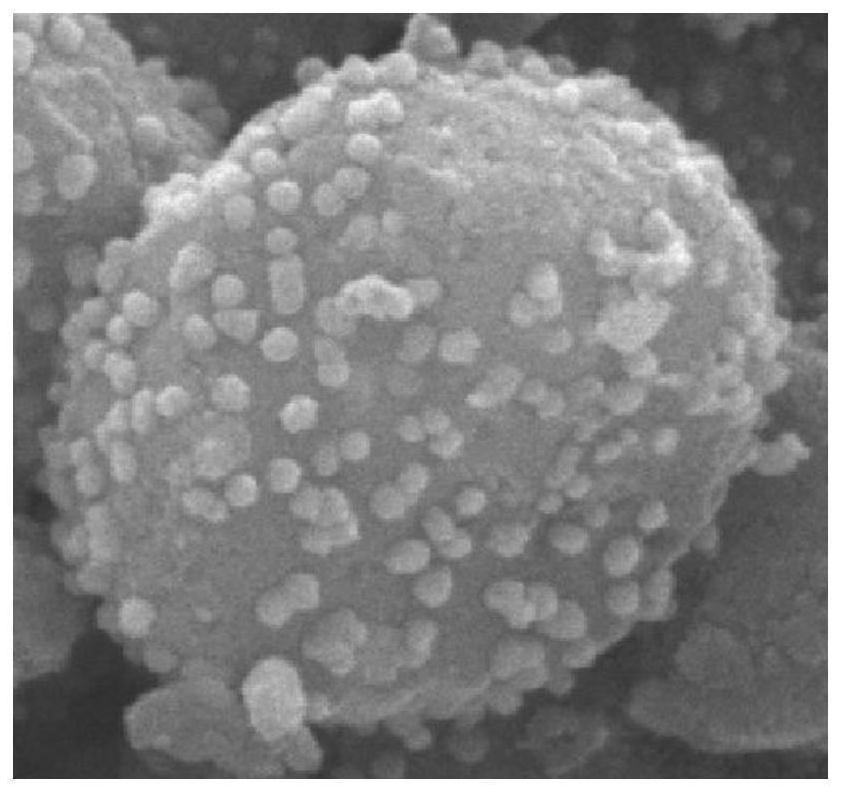
[0031] Preparation of Fe 3 o 4 @Zn-ZIF-8: Weigh 1.77g of zinc nitrate hexahydrate and dissolve it in 60.5ml of anhydrous methanol to obtain solution 1; weigh 9.85g of 2-methylimidazole and dissolve it in 60.5ml of anhydrous methanol to obtain a solution 2; After mixing solution 1 with solution 2, add 0.08g Fe 3 o 4 Disperse into the mixed liquid, stir magnetically at 50°C for 4h; after the reaction, centrifuge at 8000r.p.m, and wash with methanol three times; the obtained solid is vacuum-dried at 80°C overnight, and ground to obtain Fe3O4@Zn-ZIF-8 . Such as figure 2 Shown is Fe3O4@Zn-ZIF-8 obtained in the present invention. Example 3
Embodiment 3
[0032] Preparation of [Bmim]Br / AlBr3-Fe 3 o 4 @Zn-ZIF-8: Dissolve 0.4g of ionic liquid [Bmim]Br / AlBr3 in 5ml of anhydrous methanol, and weigh 0.63g of Fe 3 o 4 @ZIF-8 added [Bmim]Br / AlBr 3 After ultrasonic treatment for 10 minutes, seal it with plastic wrap and place it in a ventilated place for immersion for 24 hours, then separate the catalyst with a magnet, dry it in a vacuum oven at 80°C overnight, and place the solid catalyst in a muffle furnace for calcination at 220°C After grinding, the recyclable MOFs immobilized ionic liquid catalyst [Bmim]Br / AlBr3-Fe3O4@Zn-ZIF-8 was obtained. Such as image 3 Shown is [Bmim]Br / AlBr3-Fe3O4@Zn-ZIF-8 obtained in the present invention.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com