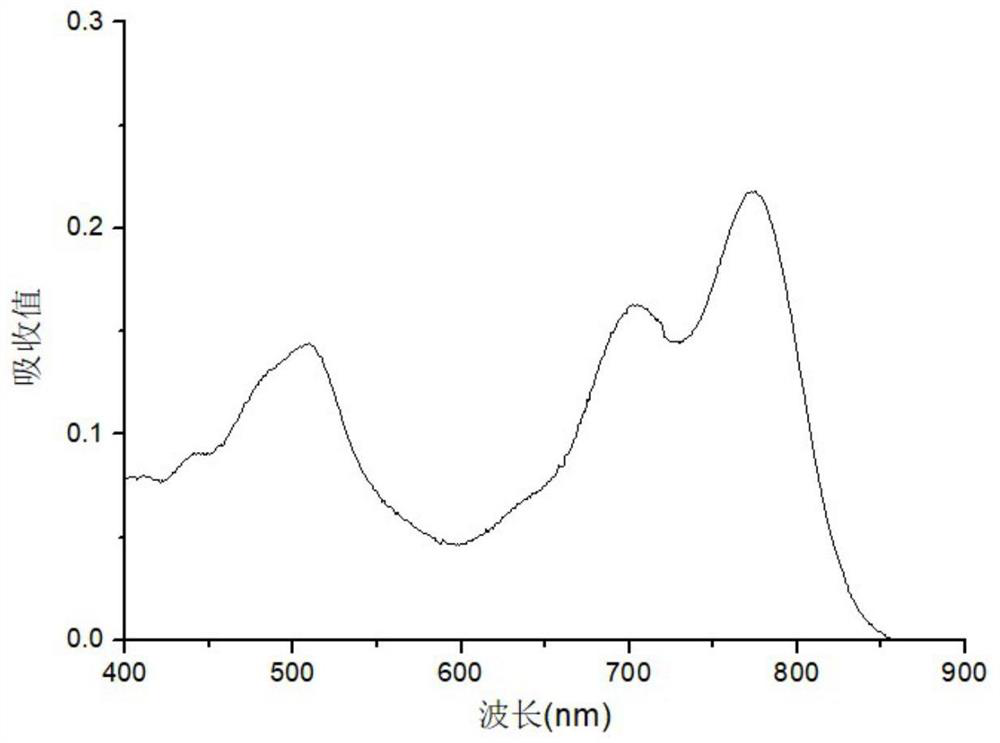
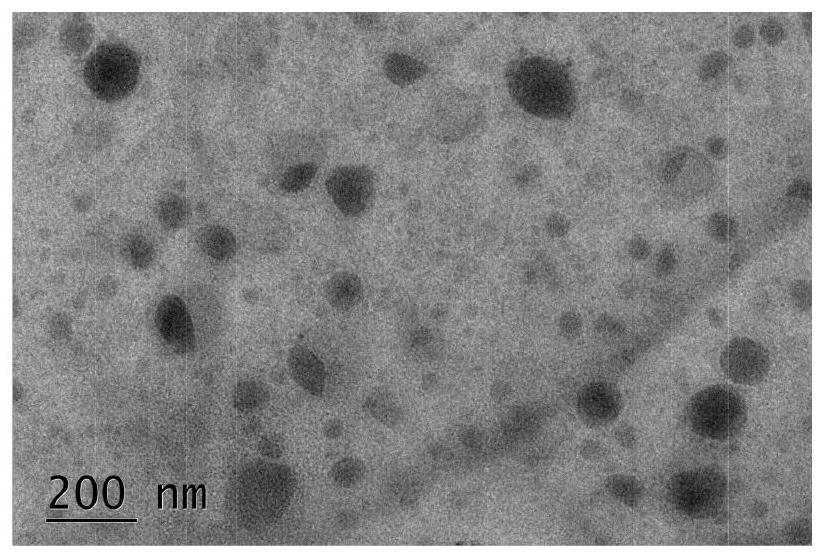
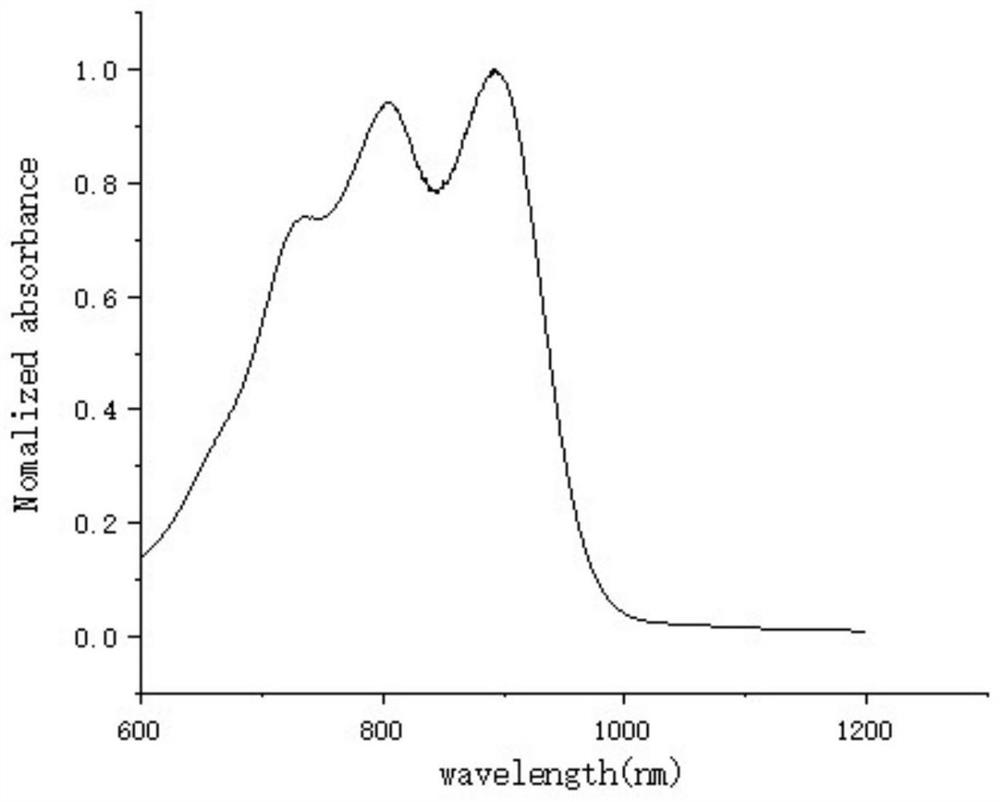
Second near-infrared window nano photosensitizer based on NDI molecules, and preparation method thereof
A photosensitizer, near-infrared technology, applied in medical preparations containing active ingredients, photodynamic therapy, chemical instruments and methods, etc., can solve the problems of lack of research and reports, reduce side effects of treatment, enhance penetration, The effect of reducing the chance of recurrence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 4.99 millimoles of 1,4,5,8-naphthalene tetracarboxylic anhydride (compound 1) and 12.5 millimoles of dibromoisocyanuric acid were catalyzed by 40 milliliters of oleum, stirred at room temperature for 3 hours, and then poured into 400 grams of In ice water, add 600 ml of water to filter to obtain a yellow precipitate, wash with 100 ml of water, and dry on potassium hydroxide to obtain tetrabromo-substituted naphthalene tetracarboxylic anhydride (compound 2).
[0036] Add 0.343 mmoles of compound 2 to 5 ml of glacial acetic acid, add 2.4 mmoles of dodecylamine, heat up to 120°C and stir for 6 hours, then cool down to room temperature and add 50 ml of water, then neutralize with saturated sodium bicarbonate, It was extracted with chloroform, dried with anhydrous sodium sulfate, rotary evaporated, and passed through a column (petroleum ether:dichloromethane=2:1) to obtain compound 3.
[0037] Under nitrogen protection, 1.2 mmoles of compound 3, 0.4 mmoles of 1,5-diaminona...
Embodiment 2
[0048] This embodiment is improved on the basis of Embodiment 1. During the reflux reaction, 0.15 mmol of N-phenylglycine was added; in the water washing process after the reflux reaction, 0.15 wt % diisopropanolamine aqueous solution was used for water washing operation. Through the above improvements, the reaction rate between compound 3 and 1,5-diaminonaphthalene can be increased without affecting the purity of the finished NDI photosensitizer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com