Preparation and application of copper electrode for electrochemical reduction of carbon dioxide
A carbon dioxide, electrochemical technology, applied in electrodes, liquid chemical plating, electrolysis process, etc., can solve the problem of different catalytic reduction performance, and achieve the effect of improving selectivity and activity, increasing deposition speed, and increasing current density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
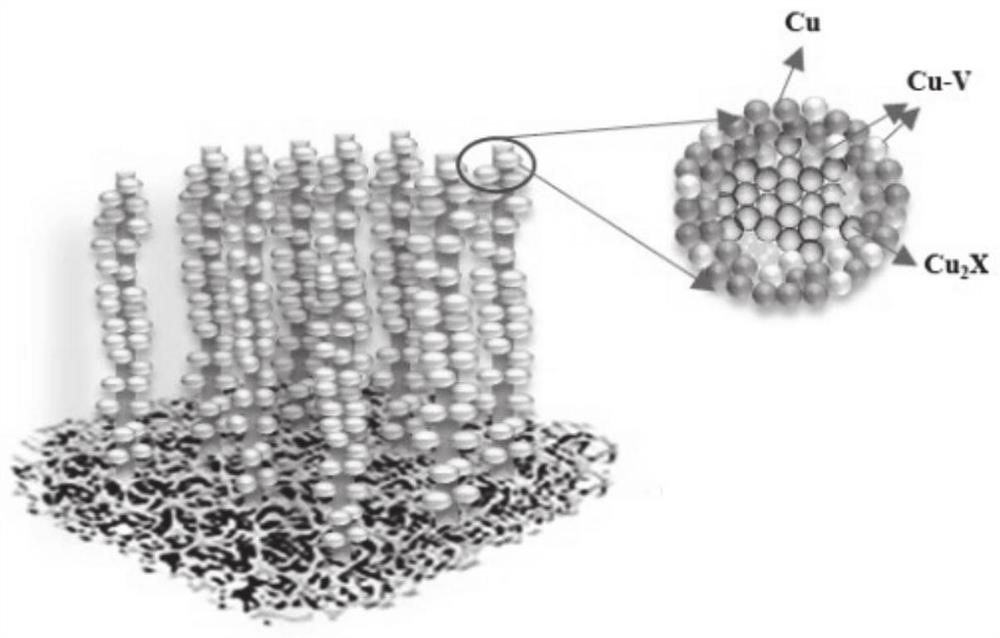
[0048] 1. Use copper mesh as the base, ultrasonically wash in water and ethanol for 10 minutes, and then wash in 0.5M H 2 SO 4 in, and at 20mA / cm 2 Perform leveling for 360s, ultrasonic cleaning, and dry under the protection of an inert atmosphere as the substrate;
[0049] 2. Place the treated base layer in a tube furnace for heat treatment for a certain period of time. The high-temperature heat treatment is carried out in air at 600°C for 24 hours to obtain Cu x O nanowires / substrate;
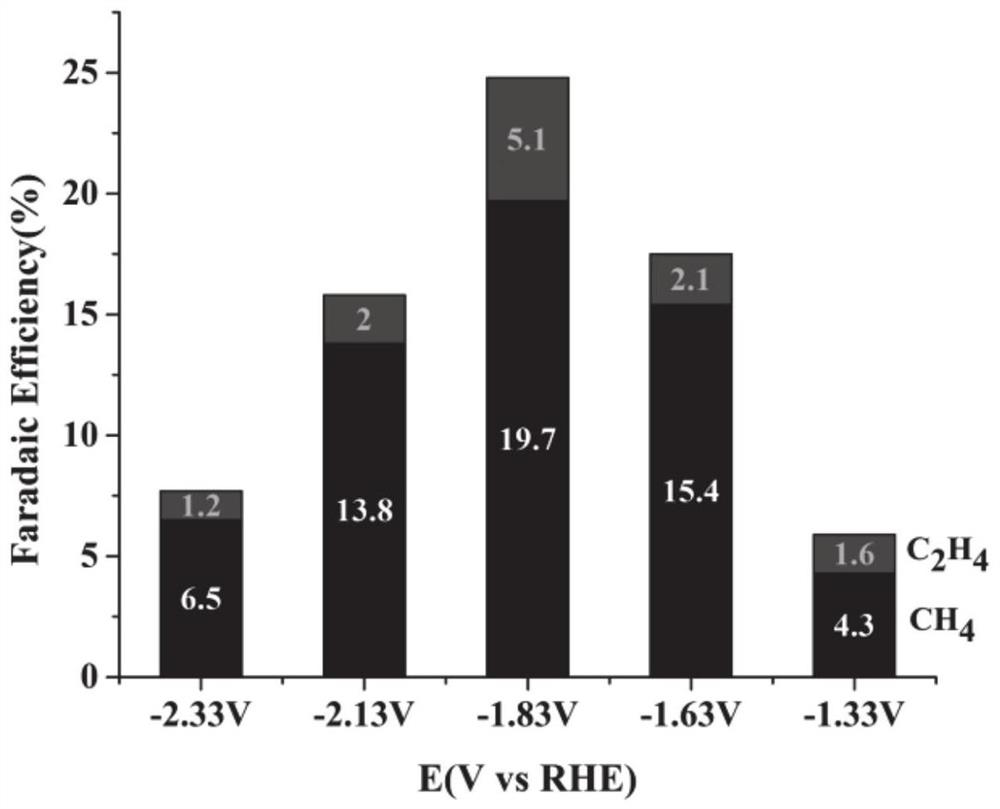
[0050] 3. Select Cu(NO 3 ) 2 ·3H 2 O is used as a precursor copper salt solution, wherein the concentration of copper ions is 7mM; a certain amount of urea is added as an alkaline precipitating agent, so that the molar concentration ratio of the alkaline precipitating agent and copper ions is 20:1, and vitamin C is added as a reducing agent. Make the molar ratio of the reducing agent to the precursor copper salt 2:1, select 200ppm of sodium saccharinate as an additive, add an alkaline p...
Embodiment 2
[0055] 1. Use copper mesh as the base, ultrasonically wash in water and ethanol for 10 minutes, and then wash in 0.5M H 2 SO 4 in, and at 20mA / cm 2 Perform leveling for 360s, ultrasonic cleaning, and dry under the protection of an inert atmosphere as the substrate;
[0056] 2. Place the treated base layer in a tube furnace for high-temperature heat treatment for a certain period of time. The high-temperature heat treatment is performed in air at 600° C. for 24 hours to obtain Cu x O nanowires / substrate;
[0057] 3. Select Cu(NO 3 ) 2 ·3H 2 O is used as a precursor copper salt solution, wherein the concentration of copper ions is 7mM; a certain amount of ammonia is added as an alkaline precipitant, so that the molar concentration ratio of the alkaline precipitant to copper ions is 20:1, and glucovitamin C is added as a reducing agent , so that the molar ratio of the reducing agent to the precursor copper salt is 2:1, select 200ppm of sodium saccharinate as an additive, ad...
Embodiment 3
[0062] 1. Use copper mesh as the base, ultrasonically wash in water and ethanol for 10 minutes, and then wash in 0.5M H 2 SO 4 in, and at 20mA / cm 2 Perform leveling for 360s, ultrasonic cleaning, and dry under the protection of an inert atmosphere as the substrate;
[0063] 2. Place the treated base layer in a tube furnace for high-temperature heat treatment for a certain period of time. The high-temperature heat treatment is performed in air at 600° C. for 24 hours to obtain Cu x O nanowires / substrate;
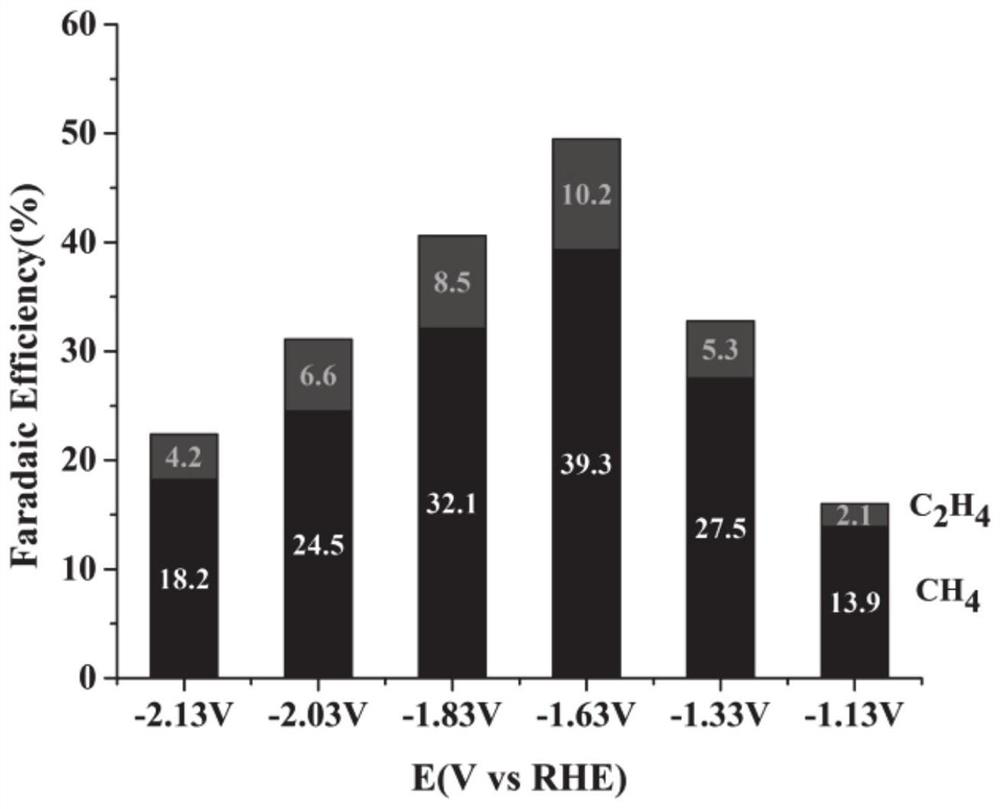
[0064] 3. Select Cu(NO 3 ) 2 ·3H 2 O is used as a precursor copper salt solution, wherein the concentration of copper ions is 7mM; a certain amount of urea is added as an alkaline precipitating agent, so that the molar concentration ratio of the alkaline precipitating agent and copper ions is 20:1, and vitamin C is added as a reducing agent. Make the molar ratio of the reducing agent to the precursor copper salt 2:1, select 400ppm of sodium tartrate as an additive, add ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com