Preparation method of biomineralized immobilized lipase and application of biomineralized immobilized lipase in catalytic synthesis of OPO
A technology for immobilizing lipase and fat, applied to biochemical equipment and methods, enzymes immobilized on or in inorganic carriers, etc., can solve the problems of poor operation and storage stability of natural enzymes, difficulties in enzyme recovery and reuse, Impact and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
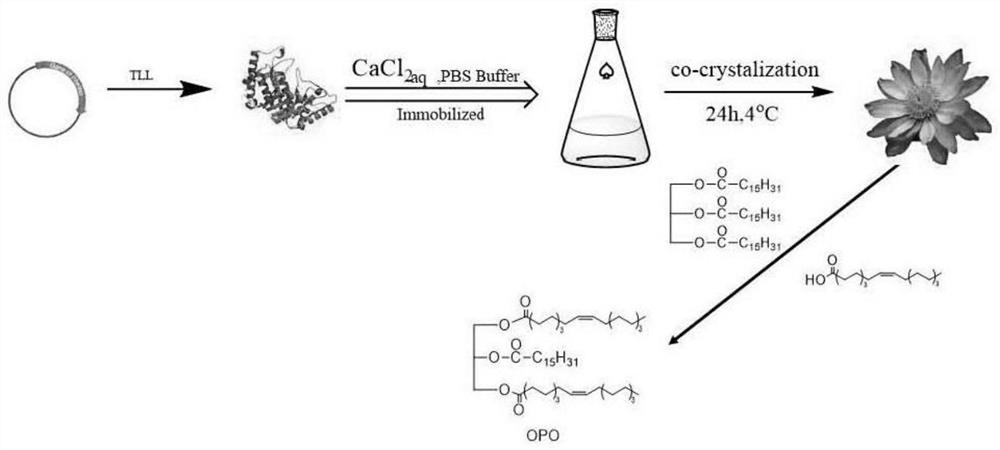
[0030] refer to figure 1 , the lipase TLL was immobilized by biomineralization, the lipase was dispersed in phosphate buffer, and a certain proportion of CaCl was added 2 solution, a white precipitate was formed, and then the mixture was left standing at 4°C to obtain immobilized lipase, which was used to catalyze the synthesis of 1,3-dioleyl-2-palmitoylglycerol (OPO).
[0031] In this invention, a simple method was used to co-produce TLL-hydroxyapatite nanoflowers (TLL@HAp-NFs) complexes with calcium phosphate through a biomineralization process, and it was applied to the synthesis of OPO, first , TLL gene was constructed on the pPIC9K vector, and then the recombinant plasmid was transferred to Pichia pastoris strain GS115, and lipase TLL was obtained after induced expression.
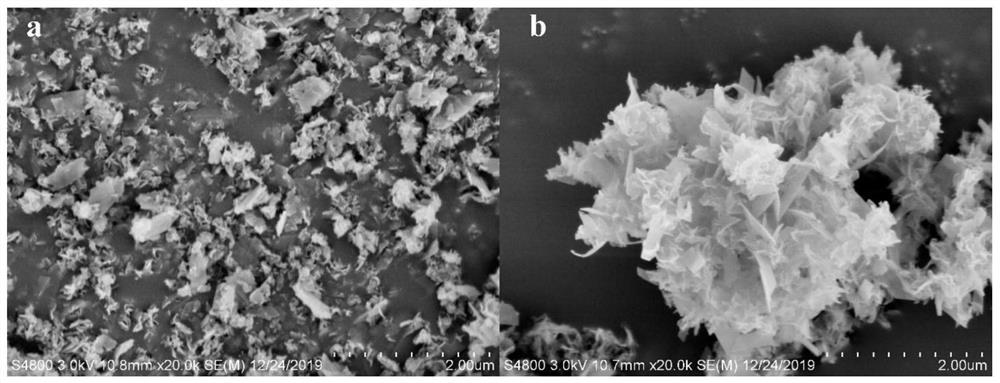
[0032] Preparation of TLL@hydroxyapatite nanoflowers (TLL@HAp-NFs)
[0033] First, the concentration of the purified and concentrated lipase protein was detected by the Bradford protein detection kit,...
Embodiment 2
[0038] Synthesis of OPO Catalyzed by TLL@Hydroxyapatite Nanoflowers
[0039] TLL@HAp-NFs were used as biocatalysts for the enzymatic acid hydrolysis of tripalmitin (PPP) and oleic acid (OA) to produce OPO. The effects of different reaction conditions on the product were studied, including water content, reaction temperature, molar ratio of reactants and so on.
[0040] The catalytic transesterification reaction is as follows: PPP (0.24mmol), OA (0.48-2.40mmol), TLL@hydroxyapatite nanoflowers (5-25mg·mL -1 ) into different solvents (6mL total volume, n-pentane, n-hexane, n-heptane, n-octane, cyclohexane and acetone) to dissolve, the water content in the reaction is 2%, the reaction temperature is 25-55°C, and The speed of 220rpm was reacted in the shaker for 12h. Solvents such as n-hexane used in the reaction need to use molecular sieves Dry for 24+ hours. Take 500 μL of each sample, and then add 500 μL of chromatographic grade n-hexane to mix. All experiments were repeat...
Embodiment 3
[0042] Effect of Reaction Temperature on Catalytic Reaction
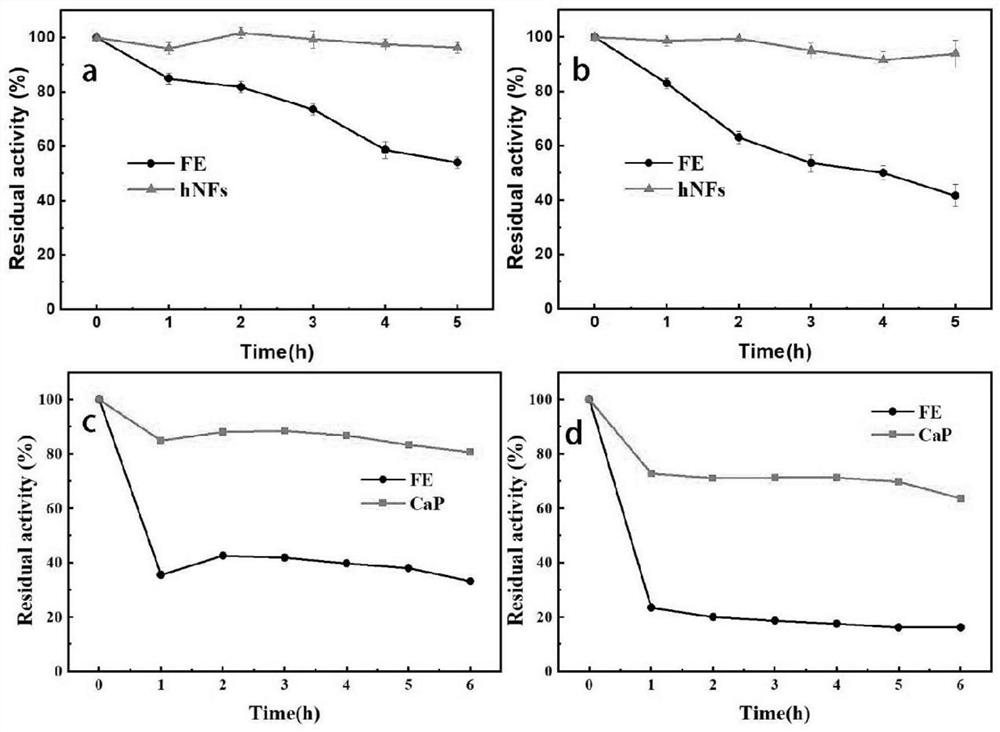
[0043] The catalytic results at reaction temperatures of 25°C, 35°C, 45°C and 55°C were mainly explored and compared. In this experiment, we found that not all reactions increased yield with increasing temperature, but free enzyme TTL was more sensitive to increasing temperature. At 25°C and 35°C, the OPO content in the reaction catalyzed by the free enzyme can be kept above 40%, but at 45°C and 55°C, the OPO content decreases significantly. However, the change of OPO content in the catalytic reaction solution of TLL@hydroxyapatite nanoflowers is relatively stable, and the OPO content is at least 35%. But too high a temperature can also negatively affect the immobilized enzyme. Analysis of its reason may be that the temperature is too high to affect the activity of the enzyme, and the temperature is too low will cause the substrate tripalmitin can not be completely dissolved in the reaction solvent, so that the en...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com