Method for preparing bridge-type tetrahydrodicyclopentadiene
A technology of tetrahydrodicyclopentadiene and dicyclopentadiene, which is applied in the field of preparing bridged tetrahydrodicyclopentadiene, can solve the problems of high operating pressure and low hydrogen utilization rate, and achieve high raw material utilization rate and ease of process , The effect of simplifying the equipment process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0009] The method for preparing bridged tetrahydrodicyclopentadiene provided by the present invention comprises: in the presence of a hydrogenation catalyst, adding dicyclopentadiene and a hydrogen-donating agent into a reactor to hydrogenate dicyclopentadiene into a bridged tetrahydrodicyclopentadiene Formula tetrahydrodicyclopentadiene, wherein the reaction temperature is 80°C-240°C, and the reaction time is 1h-24h.
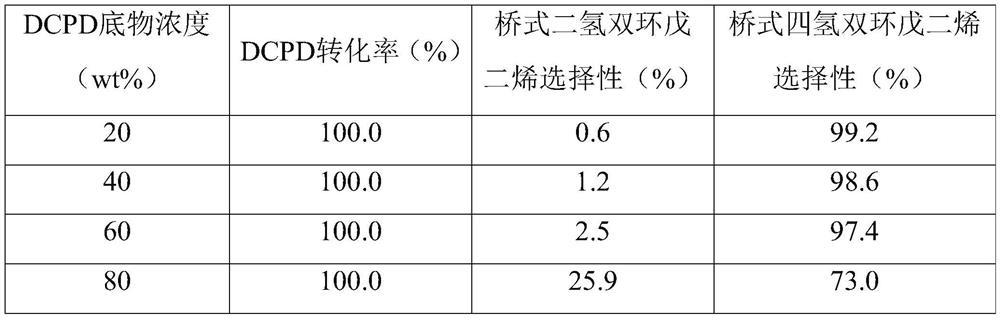
[0010] The dicyclopentadiene is dissolved in an organic solvent or an organic solvent is added to a reactor to form a solution, and the organic solvent is a medium polar oxygen-containing solvent, such as C1~C3 fatty alcohols, furans, etc., preferably methanol, Ethanol, isopropanol, tetrahydrofuran, etc. Wherein, the mass fraction of dicyclopentadiene in the solution (abbreviated as substrate concentration) is 10%-80%, preferably 20%-60%.
[0011] According to the present invention, the hydrogen-donating agent is selected from one or more of C1-C3 fatty alcoho...
Embodiment 1
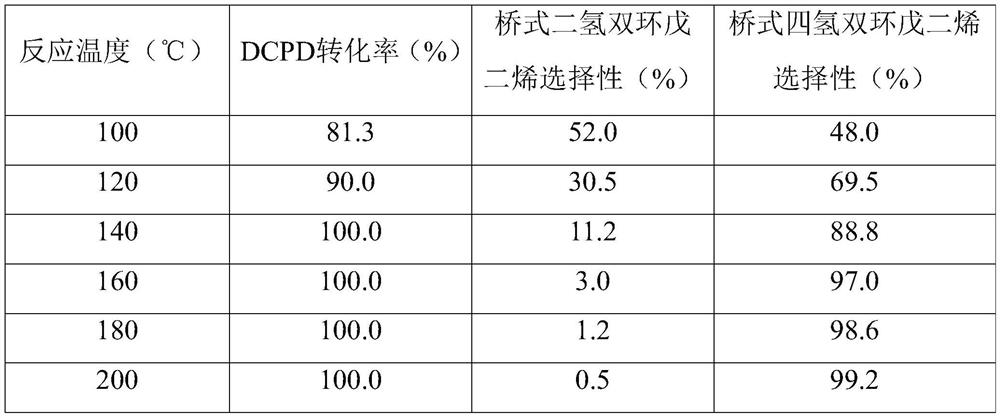
[0018] Embodiment 1 (the impact of different reaction temperatures)
[0019] With the isopropanol solution of 40wt% dicyclopentadiene as the substrate, add 25g; with 10% Pd / C as the catalyst, the amount added is 10% of the mass of dicyclopentadiene, 1g; with formic acid as the hydrogen donor, The amount of addition is 3 times the amount required for the complete saturation of DCPD in theory, 20g, and formic acid is evenly added in batches every 2h; the influence of different reaction temperatures on the conversion rate of DCPD and the selectivity of Endo-THDCPD is investigated.
[0020] Reaction conditions: Add the above-mentioned reaction raw materials, hydrogen donor reagent, into a 100mL round bottom flask, keep the stirring speed at 200rpm, stir at room temperature for 30min, then add the catalyst, heat to the reaction temperature with an oil bath, keep for 10h, and then take a sample , after filtering out catalyzer, analyze with gas chromatography, its reaction result is ...
Embodiment 2
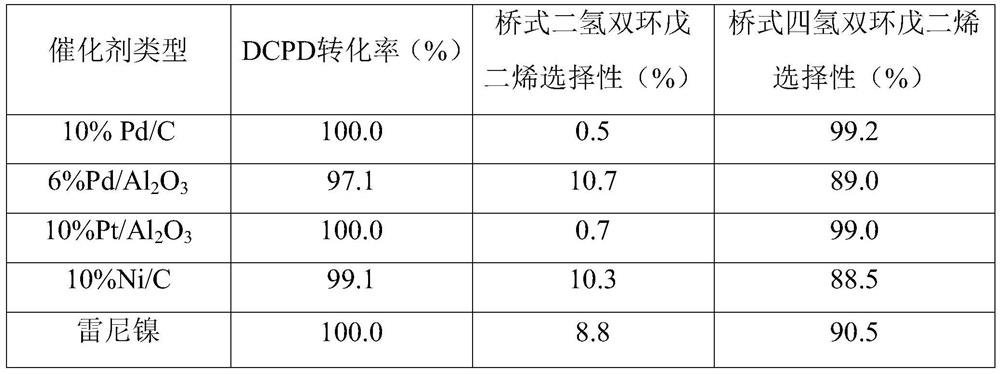
[0022] Embodiment 2 (the impact of different catalysts)
[0023] With 40wt% dicyclopentadiene in isopropanol solution as substrate, add 25g; with formic acid as hydrogen donor reagent, the amount added is 3 times the amount required for the complete saturation of DCPD in theory, 20g, and formic acid is divided every 2h Add evenly in batches; use 10% Pd / C or 6% Pd / Al 2 o 3 or 10%Pt / Al 2 o 3 Alternatively, 10% Ni / C or Raney nickel was used as the catalyst, and the amount added was 10% of the mass of dicyclopentadiene, 1 g, to investigate the influence of different types of hydrogenation catalysts on the conversion rate of DCPD and the selectivity of Endo-THDCPD.
[0024] Reaction conditions: Add the above-mentioned reaction raw materials and hydrogen donor reagent into a 100mL round bottom flask, keep the stirring speed at 200rpm, stir at room temperature for 30min, then add the catalyst, heat to 200°C in an oil bath, keep for 10h, and then take a sample , after filtering ou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com