Linker peptide mediated enzyme immobilized BaPAD catalyst, and preparation method and application thereof
A technique for immobilizing enzymes and linking peptides, which is applied in biochemical equipment and methods, botanical equipment and methods, immobilized on or in inorganic carriers, and can solve the problems of separation and purification difficulties, poor selectivity, enzymes and solid carrier surfaces Inadequate adsorption and other problems, to achieve the effect of wide application prospects and good reusability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1B
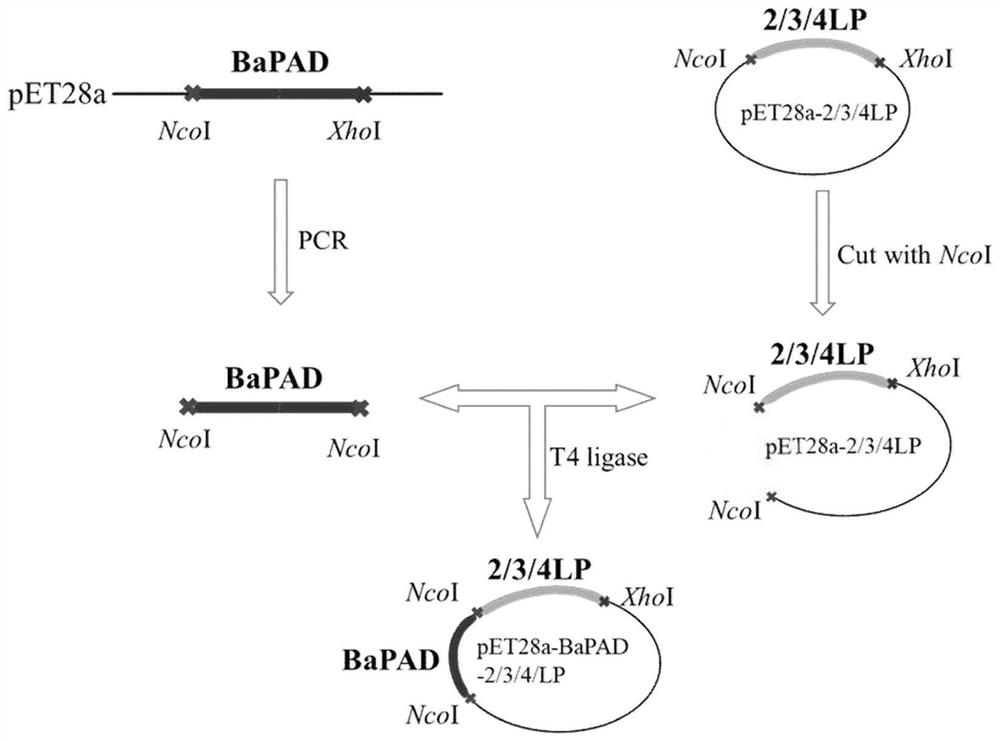
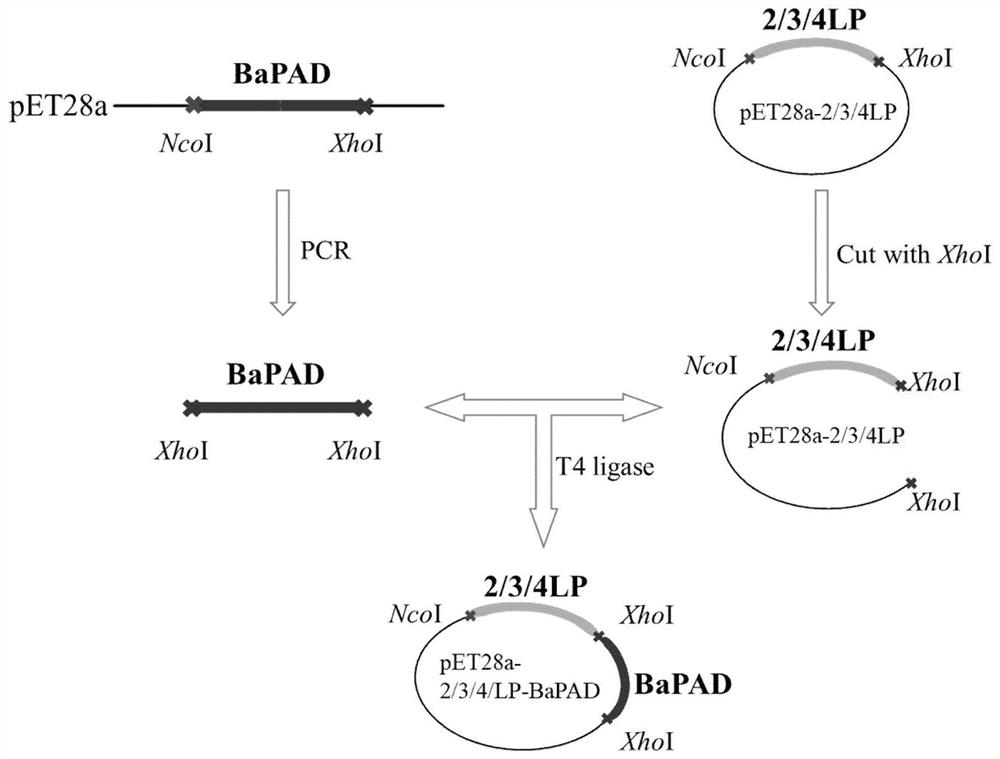
[0025] The construction of the fusion enzyme that embodiment 1BaPAD is fused with connecting peptide
[0026] (1) Construction of pET28a-bapad-lp and pET28a-lp-bapad
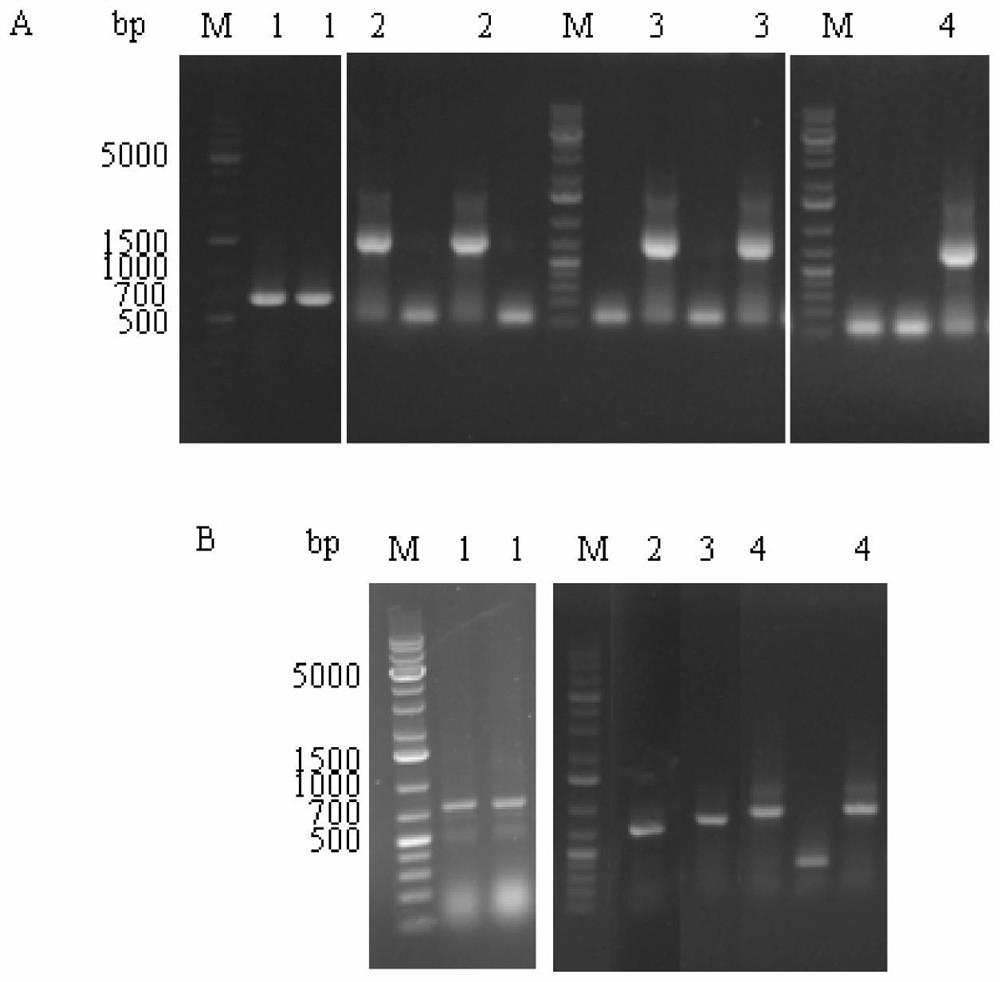
[0027] Using pET28a-bapad as a template, BaPAD-F, BaPAD-LP-R1, BaPAD-LP-R2 and LP-BaPAD-F1, LP-BaPAD-F2, BaPAD-R (Table 1) were used as primers for PCR respectively. The system is: 5×fastPfu buffer 10μL, dNTP (2.5mM) 4μL, pET28a-bapad 1μL, BaPAD-F or LP-BaPAD-F1 1μL, BaPAD-LP-R1 or BaPAD-R1μL, fastPfu DNA polymerase (10U / μL) 1 μL, ddH 2 O 32 μL. Mix the reaction system well and put it into the PCR instrument. Set the reaction conditions as follows: 1.5min pre-denaturation at 95°C, 30s denaturation at 94°C, 30s annealing at 57°C, 2min extension at 72°C, a total of 30 cycles; finally 10min at 72°C . PCR products were detected by 1% agarose gel electrophoresis. The bapad-lp and lp-bapad gene fragments containing NcoI / XhoI restriction sites were recovered, purified and amplified using Beijing Trans Gen Company'...
Embodiment 2B
[0043] Expression and purification of embodiment 2 BaPAD and fusion enzyme recombinant protein
[0044] (1) Expression and purification of recombinant protein
[0045] Select the recombinant plasmid with correct sequencing to transform Escherichia coli BL21 (DE3) competent, the method is the same as transforming top10 competent. Spread LBK plates containing 50 μg / mL kanamycin and culture overnight in a 37°C incubator.
[0046] Pick a single white colony from the overnight cultured LBK plate and place it in 3 mL of LBK liquid medium containing 50 μg / mL kanamycin, and culture it overnight at 37°C on a shaker at 200 rpm.
[0047] Inoculate the 2% inoculum of the overnight culture into 50mL sterilized LBK liquid medium, and cultivate it to OD at 37°C with a shaker at 200rpm 600 is 0.6.
[0048] Add IPTG with a final concentration of 0.4mM, induce expression culture at 28°C, 200rpm shaker, and after 12h, centrifuge the bacterial solution at 8500rpm for 10min to collect the bacte...
Embodiment 3
[0057] Example 3 Adsorption experiments of BaPAD and fusion enzymes on different carriers
[0058] (1) Affinity adsorption of BaPAD and fusion enzymes to different carriers
[0059] Pretreatment of solid support: 10 mg of solid support was washed three times with 800 μL wash buffer (10 mM Tris-HCl, 100 mM NaCl, 1% Triton X-100, pH 7.5), and then washed with citric acid-NaCl 2 HPO 4 Buffer (200mM, pH 7.0) was washed three times, each process was vortexed, and centrifuged at 8,500rpm for 3min to remove the supernatant. Immobilization: 9 pure enzymes (50 μg, 100 μL citrate-Na 2 HPO 4 Buffer) was added to the washed carrier and incubated for 1 h with shaking on ice.
[0060] Calculation of enzyme load: After incubation, the sample was centrifuged at 8,500 rpm for 3 minutes, and the supernatant was separated. The protein concentration of the supernatant before immobilization and after adsorption was detected by BCA method, and the concentration of immobilized enzyme was obtaine...
PUM
| Property | Measurement | Unit |
|---|---|---|
| load ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com