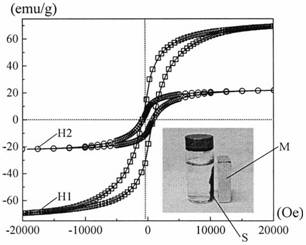
Preparation method of cerium dioxide-zinc oxide-bismuth oxyhalide-cobalt ferrite magnetic visible-light-induced photocatalyst
A technology of ceria and bismuth oxyhalide, applied in the direction of physical/chemical process catalysts, chemical instruments and methods, separation methods, etc., can solve the problems of low activity, high cost, limited adsorption capacity, etc., and achieve high photocatalytic oxidation activity , Strong magnetic recovery ability, simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
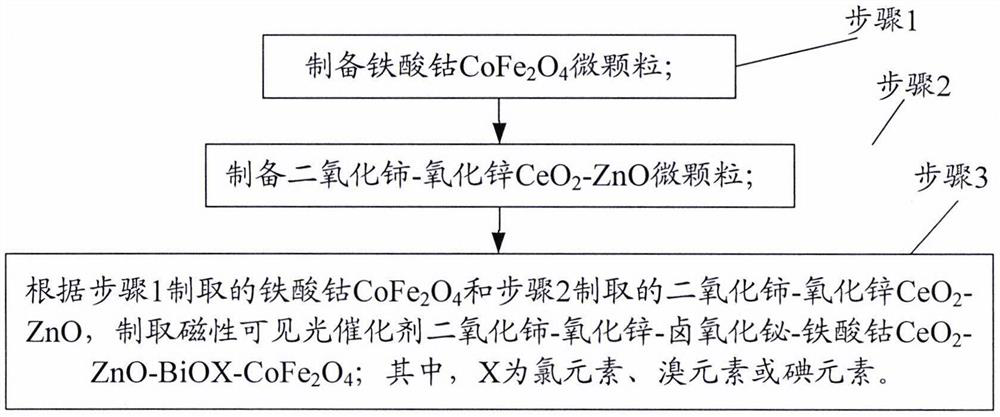
[0020] figure 1 It is an overall schematic flow chart of the preparation method of the ceria-zinc oxide-bismuth oxyhalide-cobalt ferrite magnetic visible light catalyst of the present invention. like figure 1 As shown, the preparation method of ceria-zinc oxide-bismuth oxyhalide-cobalt ferrite magnetic visible light catalyst of the present invention comprises the following steps:
[0021] Step 1, preparation of cobalt ferrite CoFe 2 O 4 microparticles.
[0022] Step 2, preparation of ceria-zinc oxide CeO 2 - ZnO microparticles.
[0023] Step 3, cobalt ferrite CoFe produced according to step 1 2 O 4 and the cerium dioxide-zinc oxide CeO produced in step 2 2 -ZnO, preparation of magnetic visible light catalyst ceria-zinc oxide-bismuth oxyhalide-cobalt ferrite CeO 2 -ZnO-BiOX-CoFe 2 O 4 ; Wherein, X is chlorine element, bromine element or iodine element.
[0024] In a word, the preparation method of ceria-zinc oxide-bismuth oxyhalide-cobalt ferrite magnetic visible li...
Embodiment
[0048] Figure 5 It is a schematic diagram of the overall structure of the experimental bench used in the embodiment of the preparation method of the ceria-zinc oxide-bismuth oxyhalide-cobalt ferrite magnetic visible light catalyst according to the present invention. like Figure 5 As shown, the experimental bench used in the embodiment of the present invention includes: a steel cylinder 1 used as the original flue gas source, a constant temperature water bath equipped with an elemental mercury permeation pipe to add mercury to the original flue gas source part from the steel cylinder 1 3. The mixer 4 for mixing the original flue gas with the mercury-containing flue gas, the flow meter 2 for controlling the gas flow rate of the original flue gas from the cylinder 1 to the constant temperature water bath 3 and the mixer 4, and the mixer 4 outputs Under the control of the flow meter 2, the flue gas to be treated enters the magnetic stirring water bath photocatalytic reactor 5 c...
Embodiment 1
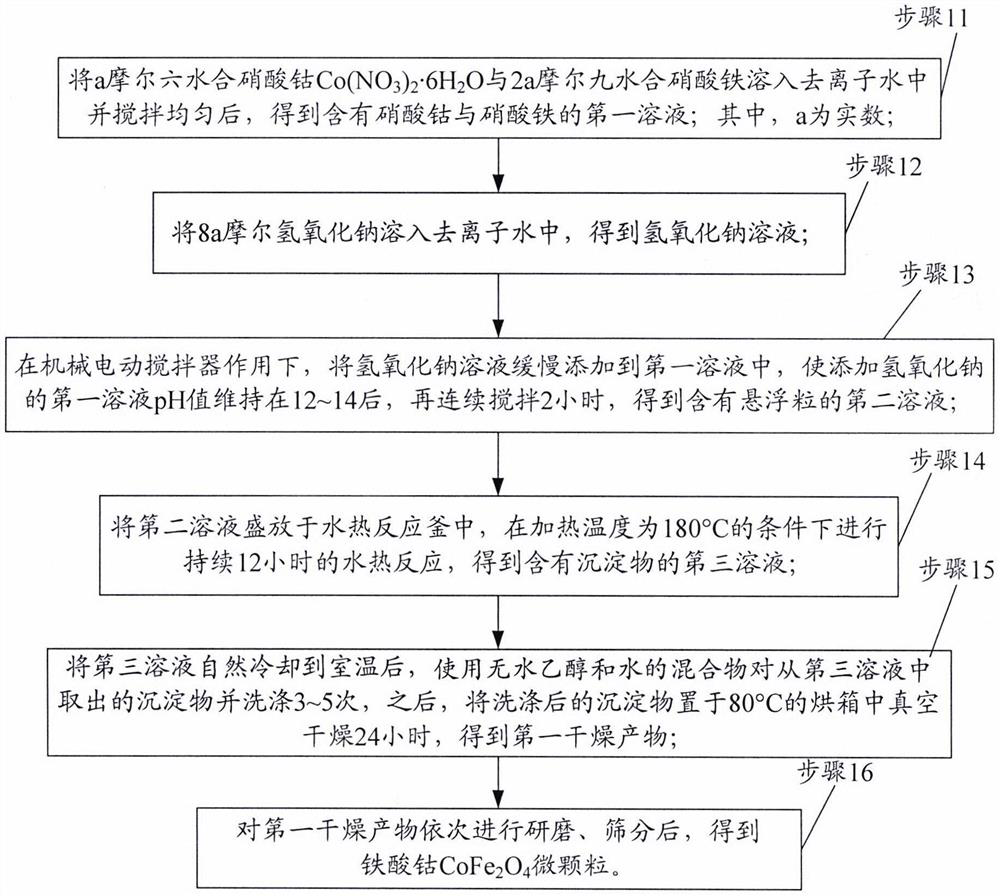
[0052] In the first embodiment, cobalt ferrite CoFe is prepared 2 O 4 Microparticles, including the following steps:
[0053] In step A11, 0.0426 mol of Co(NO 3 ) 2 ·6H 2 O and 0.0852 mol Fe (NO 3 ) 3 ·9H 2 O was dissolved in 100 mL of deionized water and stirred uniformly to obtain a first solution containing cobalt nitrate and ferric nitrate.
[0054] Step A12: Dissolving 0.35 mol of sodium hydroxide in 100 mL of deionized water to obtain a sodium hydroxide solution.
[0055] Step A13, under the action of mechanical electric stirring, slowly adding the sodium hydroxide solution to the first solution, so that the pH value of the first solution added with sodium hydroxide is maintained between 12 and 14, and stirring continuously for 2 hours to obtain a solution, A second solution containing suspended particles of ferric hydroxide and cobalt hydroxide is obtained.
[0056] In step A14, the above-mentioned second solution is placed in a hydrothermal reaction kettle, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com