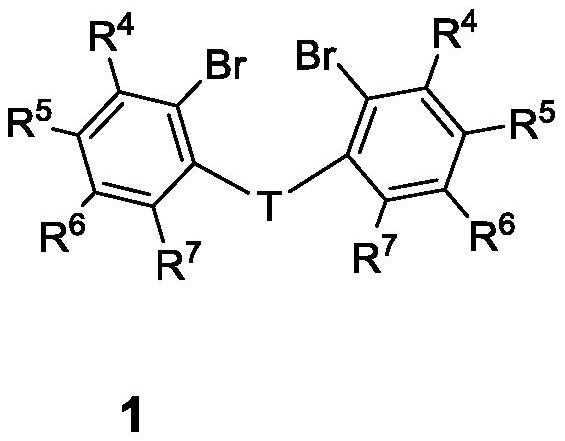
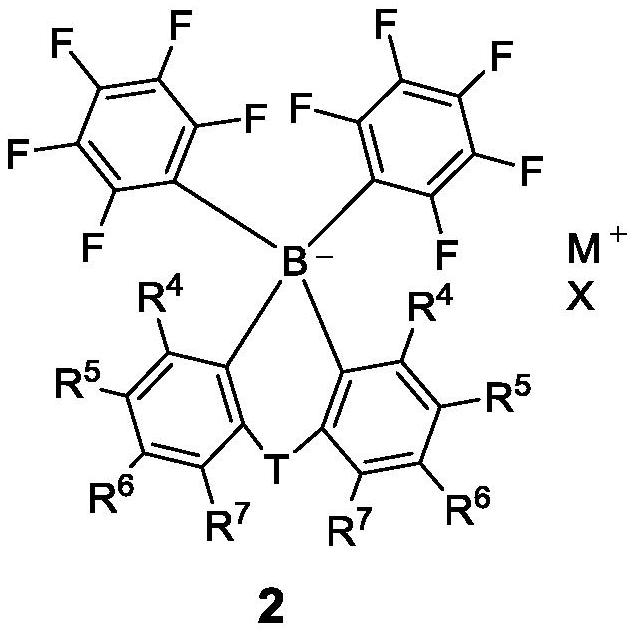
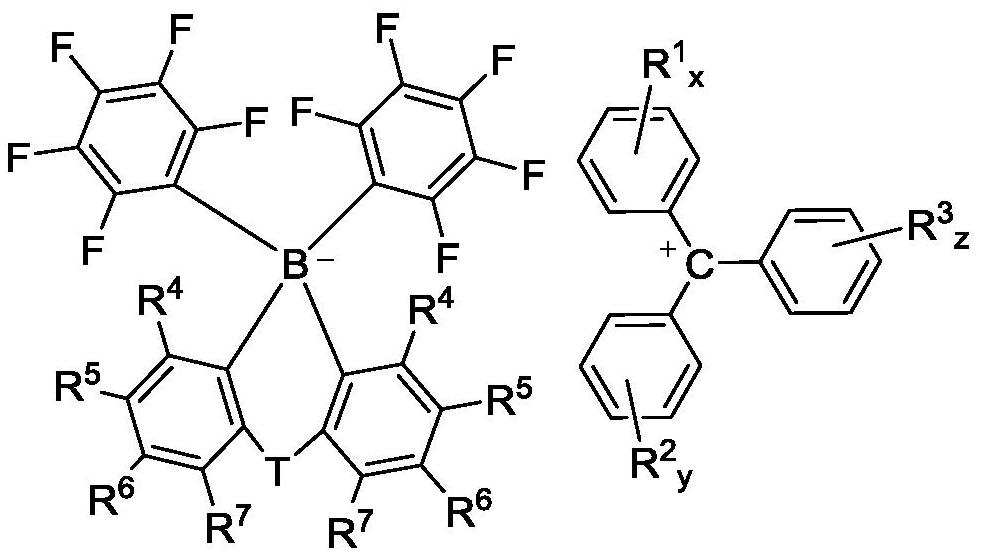
High-solubility triaryl carboborate as well as preparation method and application thereof
A triarylcarboborate, high solubility technology, applied in the field of high solubility triarylcarboborate and its preparation, can solve the problems of increased separation cost, high price, and rare reports on the development of efficient organoboron
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1, the preparation of compound 3
[0046]
[0047] (1) Under the standard schlenk technique, add 15.6 g of 2,2'-dibromobiphenyl (0.05 mol) into the reaction flask, add 100 mL of toluene as a solvent, and start stirring. Then, 62.5 mL of butyllithium hexane solution (0.1 mol, 2 eq., 1.6 mol / L) was added to the solution at -78°C, slowly raised to room temperature, and stirred for 48 h. Then, 52.5 mL of boron tribromide dichloromethane solution (0.0525 mol, 1.05 eq., 1 mol / L) was added at -78 ° C, and after the addition was completed, it was raised to room temperature and stirred for 12 h. After the reaction, the solvent was sucked dry. Then, 150 mL of toluene was added to the reaction flask, and then 17.4 g of (pentafluorophenyl) lithium (0.1 mol, 2 eq.) was added, and the reaction was stirred for 8 h. After the reaction, add an appropriate amount of water to stir, and then stand still to separate the liquids to obtain the upper layer (toluene layer) clear ...
Embodiment 2
[0051] Embodiment 2, the preparation of compound 4
[0052]
[0053] (1) Under a nitrogen atmosphere, accurately weighed 1,2-dibromotetrafluorobenzene (61.57 g, 0.2 mol) and 200 mL of anhydrous tetrahydrofuran were added into a dry two-neck round bottom flask. Then the solution was cooled to -78°C, and 125 mL of n-hexane solution of butyllithium (0.2 mol, 1.6 mol / L, 1 eq.) was added dropwise under vigorous stirring. After the dropwise addition, continue to stir at this temperature for 10 min and then slowly rise to room temperature, then add 21.6 g of 1,4-dibromobutane (0.1 mol, 0.5 eq.) and stir overnight. After the reaction was completed, 25mL of 5% hydrochloric acid solution was added to the solution, the organic layer was separated, the aqueous layer was extracted with ether (20mL×4), the organic layers were combined and washed with anhydrous MgSO 4 dry. The solvent was spun off with a rotary evaporator to obtain an off-white solid. Recrystallized with absolute ethan...
Embodiment 3
[0058] Embodiment 3, the preparation of compound 5
[0059]
[0060](1) Under the condition of anhydrous and anaerobic nitrogen blowing reflux, add 60mL of 3-chlorophenylmagnesium bromide THF solution (0.03mol, 0.5mol / L) to the reaction flask at room temperature and then start stirring, slowly 1.18 g of diethyl carbonate (0.01 mol, 1 / 3 eq.) was added dropwise, and after the addition was complete, the reaction bottle was placed in an oil bath and refluxed at 80° C. for 12 h. After the reaction is completed, cool to room temperature, then add 60 mL of octadecylmagnesium chloride THF solution (0.03 mol, 0.5 mol / L, 1 eq.) dropwise to the reaction solution, stir for 4 hours, and dropwise add saturated ammonium chloride aqueous solution, a large amount of magnesium hydroxide precipitated immediately. Continue to drop the solution until the layers are separated and the upper layer is clear. Separate the supernatant, dissolve the lower layer with dilute hydrochloric acid, and extra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com