Organic light-emitting compound and preparation method and application thereof
A light-emitting compound, compound technology, applied in the direction of organic chemistry, chemical instruments and methods, light-emitting materials, etc., to achieve the effect of strong effect, strong electron donating ability, and improving solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
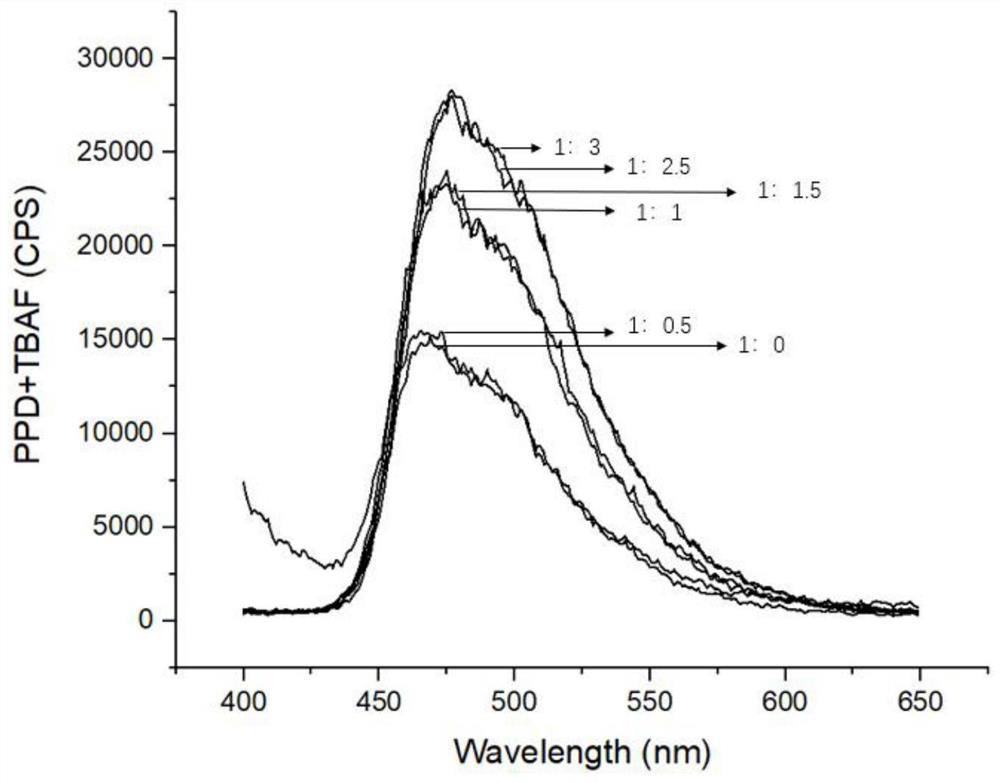
Method used
Image
Examples
preparation example Construction
[0038] The second aspect of the present invention provides a method for preparing an organic light-emitting compound, comprising the following steps:
[0039] S1: under nitrogen, using potassium tert-butoxide as a catalyst, dissolving p-toluenesulfonylmethyl isonitrile and diethyl fumarate in anhydrous tetrahydrofuran, and reacting at room temperature for 8h to 14h to obtain compound a; Wherein, the molar ratio of p-toluenesulfonylmethyl isonitrile, diethyl fumarate and potassium tert-butoxide is 1:(1-1.4):(2-2.4).
[0040] S2: The compound a is hydrolyzed in the presence of the first base, and then acidified to a pH less than 4 to obtain the compound b. Specifically, the hydrolysis process is to add compound a to a mixed solution pre-configured by the first base, ethanol and water, and reflux for 1h-3h. After adding ethanol, the solubility of compound a can be improved, which is convenient for improving the yield of compound b. In the pre-configured mixed solution of the fi...
Embodiment 1
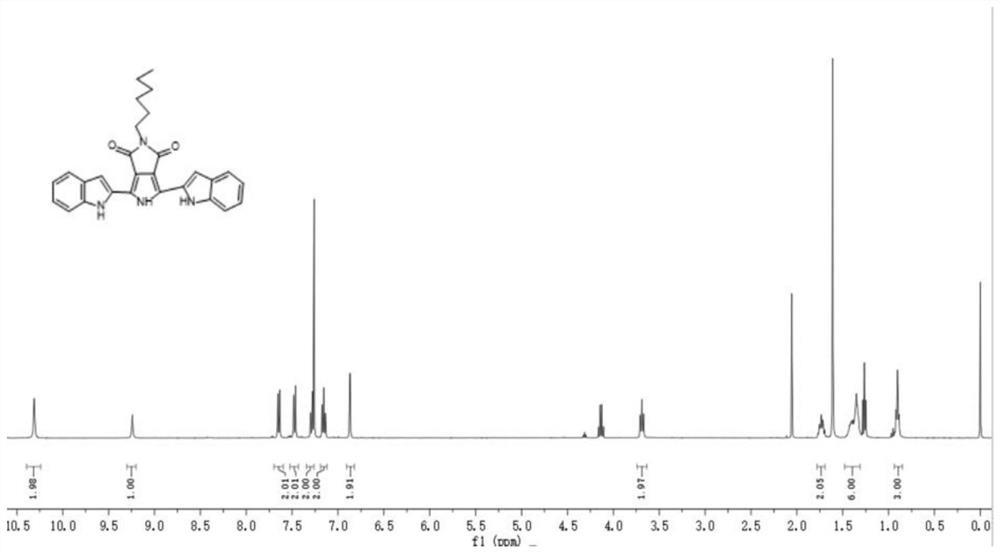
[0052] This embodiment provides an organic light-emitting compound, the synthetic route is as follows:
[0053]
[0054] Specifically prepared by the following steps:
[0055] Add 100ml of anhydrous tetrahydrofuran (THF) in a three-neck round bottom flask, add potassium tert-butoxide (11.5g, 102.6mmol) under nitrogen atmosphere, and then add p-toluenesulfonylmethyl isocyanide (10.0g, 51.2mmol) dropwise ), diethyl fumarate (8.8g, 51.2mmol) and 50mlTHF are configured as a mixed solution, then stirred and reacted at room temperature for 12h, added saturated brine after the reaction was completed, and extracted the organic layer with 50ml of THF, dewatered After filtering and removing the solvent, it was dissolved in methanol; water was added to the methanol solution until a precipitate was formed, cooled, filtered to obtain a solid, and rinsed and dried with excess water to obtain milky white compound a (6.4g, yield 59%) .
[0056] Compound a (5.4g, 25.6mmol) was added to a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com