Condensed ring aromatic amine compound used in organic layer of OLED device as well as synthesis method and application of condensed ring aromatic amine compound
A technology of aromatic amines and compounds, which is applied in the field of fused-ring aromatic amine compounds and their synthesis, can solve problems such as low device efficiency, interface degradation, and poor stability, and achieve stable performance, increased steric hindrance, and long life. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
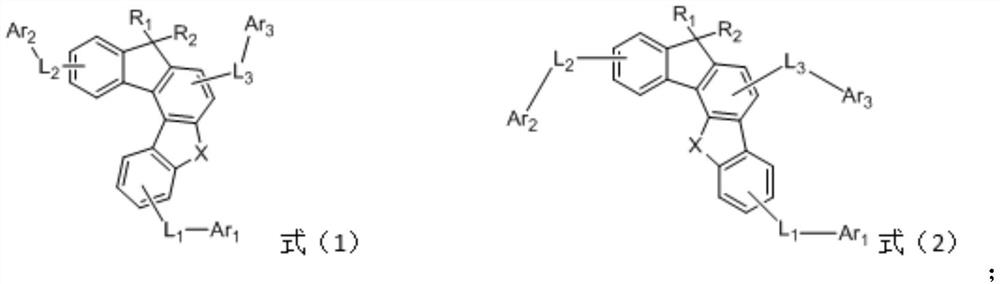
[0094] Specifically, when the condensed ring aromatic amine compound is the general formula (2), its synthesis method includes the following steps:
[0095] N1, dibenzofuran-4-boronic acid or its derivatives, 2-bromo-4-chloro-1-iodobenzene or its derivatives and Pd(Ph 3 P) 4 Suspended in a solvent, adding potassium carbonate solution, reflux reaction to obtain the intermediate product c,
[0096]
[0097] N2, in an inert atmosphere, at -78°C, dissolve the intermediate product c in a solvent, add n-BuLi, The reaction at room temperature gives the intermediate product d,
[0098]
[0099] N3, in an inert atmosphere, the intermediate product d and amine compounds are dissolved in a solvent, and tri-tert-butylphosphine solution, Pd 2 (dba) 3 And sodium tert-butoxide, heated to reflux reaction, obtains the compound shown in formula (2);
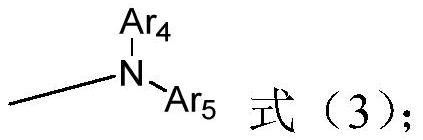
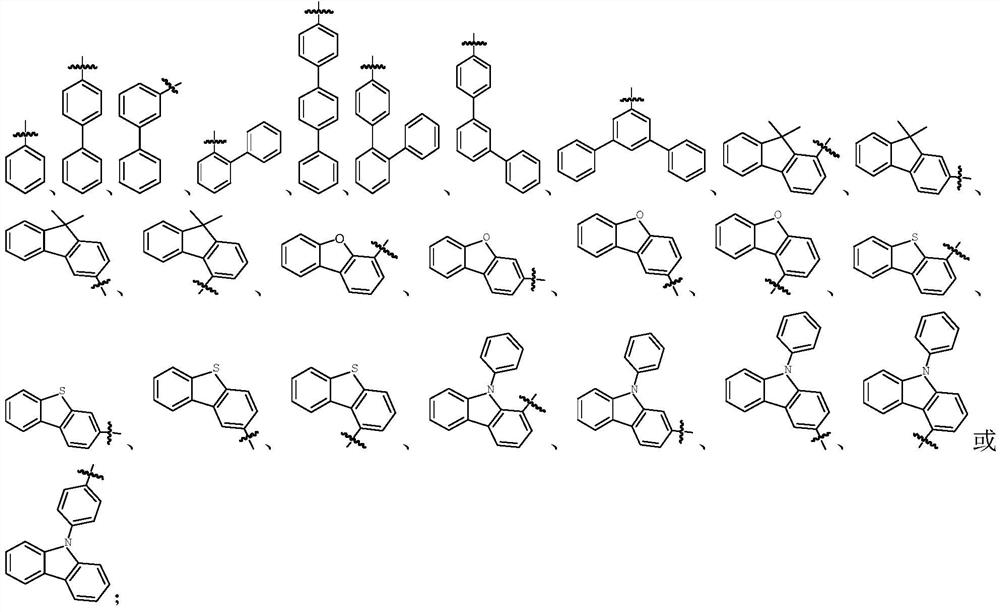
[0100] Among them, R 1 , R 2 , X, L 1 , L 2 and L 3 The represented group is the same as in the above-mentioned formula (1) or f...
Synthetic example 1
[0132] Synthesis of intermediate product a-1
[0133] 100g (462mmol) of dibenzofuran-1-boronic acid, 138g (438mmol) of 2-bromo-4-chloro-1-iodobenzene and 10.7g (9.2mmol) of Pd(Ph3P)4 were suspended in 980ml of dioxin Add 1000ml of 2M potassium carbonate solution slowly to this suspension in alkanes, and reflux for 16h. After the reaction is completed, cool to room temperature, separate the organic phase, wash with water for 3 times and spin dry the organic solvent by rotary evaporation. The silica gel column obtained the intermediate product a-1, and the yield was 66.9g 43%;
[0134] Synthesis of intermediate product b-1
[0135] Put 35.5g (100mmol) of intermediate product a-1 into a dry reaction flask, inject 300ml of anhydrous THF into the reaction flask with a syringe, pump nitrogen for 3 times, stir at -78°C for half an hour, and pour into the reaction flask Add 50ml of n-BuLi (2M ethane solution) dropwise, and after stirring for 1 hour, add 21.8g (120mmol) benzophenone ...
Synthetic example 2
[0143] The synthesis procedure of compound 1-2 in Synthesis Example 2 is basically the same as that of compound 1-1, except that 7.9 g (22 mmol) of biphenyl-4-yl-(9,9-dimethyl-9H-fluoren-2-yl) is used Amine replaces bis-benzidine, and the obtained compound 1-2 has a quality of 10.4 g, a yield of 68%, and a molecular formula of C 58 h 41 NO, the relative molecular mass is 767.32; the structural characterization data of compound 1-2 are as follows: EA: C, 90.71; H, 5.38; N, 1.82;
[0144]1HNMR: 8.20ppm(1H); 7.98ppm(1H); 7.9-7.86ppm(2H); 7.75ppm(2H); 7.69ppm(1H); 7.55-7.54ppm(4H); 7.52ppm(1H); 7.49ppm (2H); 7.30-7.41 (8H); 7.25-7.30ppm (6H); 7.18-7.16ppm (3H); 7.10-7.08ppm (4H); 1.65ppm (6H).
[0145]
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com