Preparation method and application of novel halophilic archaea extracellular protease
A technology of extracellular protease and halophilic archaea, applied in the field of genetic engineering, can solve the problem that protease cannot adapt to high salinity industrial processing environment, etc., and achieve the effect of excellent enzymatic characteristics, good stability and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Acquisition of hly encoding gene hly for novel halophilic archaeal extracellular protease;
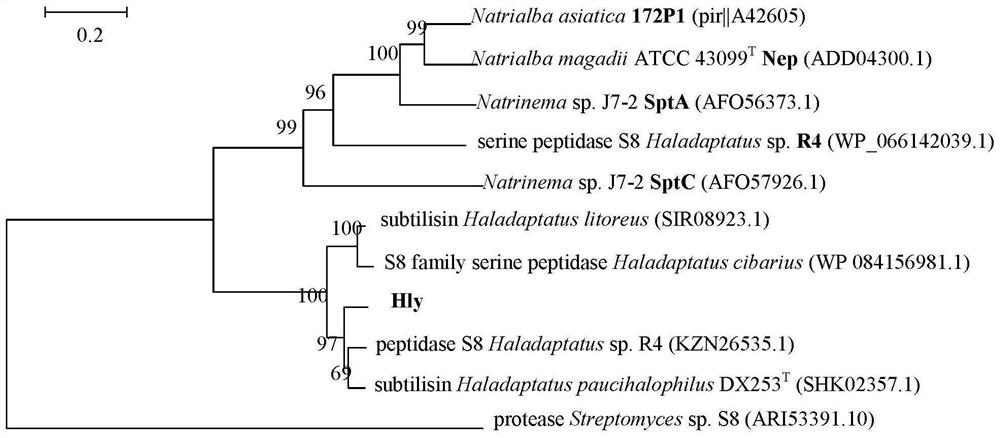
[0057] Based on a halophilic archaea Haladaptatus sp.DYF 46 (separated by the inventor from Dongying Salt Field, Shandong, China, preserved in China General Microorganism Culture Collection and Management Center, patent strain preservation number: CGMCC 19759, preservation date: 2020.4.29) The whole genome, open reading frame prediction and gene annotation results were used to screen suspected extracellular protease genes. The consistency between the sequence and the known hydrolase gene sequence in the database was compared by Blastp (http: / / blast.ncbi.nlm.nih.gov / ). The hly gene obtained through database comparison analysis has a size of 1191bp and a base composition of 238A (19.98%), 176T (14.78%), 395C (33.17%) and 383G (32.16%), and its nucleotide sequence is as shown in SEQ ID No. :1 shown. The size of the encoded protein is 396 amino acid residues, and its amino acid se...
Embodiment 2
[0061] Construction and induced expression of recombinant expression plasmid of hly;
[0062] The gene hly obtained in the present invention is cloned into an expression vector, and a recombinant expression strain is constructed for inducible expression. Based on the ORF analysis of NCBI ORF Finder, the gene open reading frame sequence was obtained, and the upstream primer hly-NcoI-F (5'-ATAccatggCAAGGAAAGCCAATGGCG-3', NcoI) and the downstream primer hly-XhoI-R (5'- ATActcgagGTTGTCGCTGGAGTCGAG-3', XhoI), PCR amplification to obtain the target fragment (1206bp). The expression plasmid was constructed by restriction endonuclease cloning, that is, the PCR product was double-digested with restriction enzymes NcoI and XhoI, and the purified fragment was ligated with the plasmid pET28a that had been double-digested with NcoI and XhoI to obtain the recombinant plasmid pET28a-hly. The 3' end of the insert (NcoI and XhoI sites) contains 6×His tag. CaCl 2 The transformation method wa...
Embodiment 3
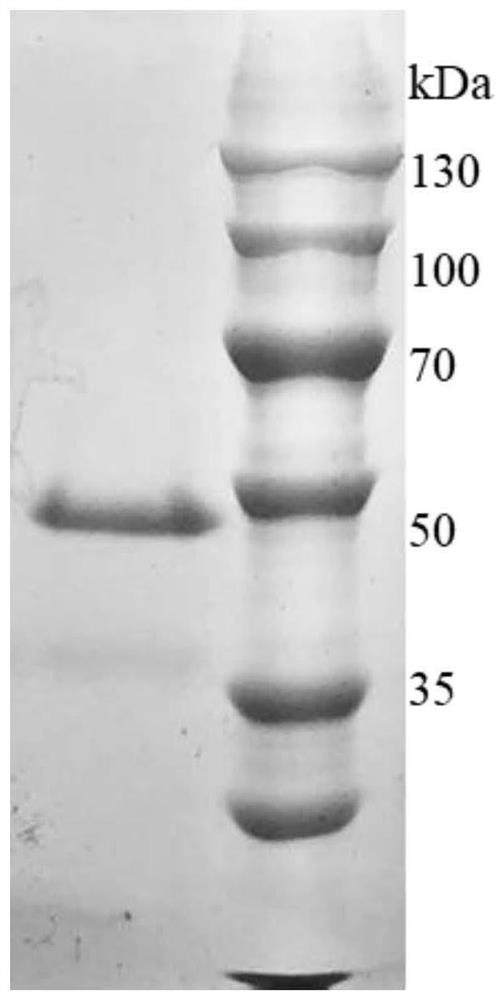
[0065] Purification and refolding of recombinant protein Hly;
[0066] Resuspend the bacteria collected by low temperature centrifugation in 30mL cell lysate (8M urea, 10mM CaCl 2 , 50 mM Tris-HCl, pH 8.0) and sonicated on ice. The supernatant was collected by low temperature centrifugation. The mature enzyme was obtained from the supernatant by the on-column renaturation method (that is, the protein was purified by nickel affinity chromatography and the protease was renatured in vitro in a high-salt environment). The specific operation is as follows:
[0067] 1) Add β-mercaptoethanol with a final concentration of 2mM to the cell lysate of 10 times the column bed volume (CV), and equilibrate the nickel column.
[0068] 2) Add 2mM final concentration of β-mercaptoethanol to the supernatant and load the sample.
[0069] 3) Add 5CV buffer I (2mM β-mercaptoethanol, 8M urea, 40mM imidazole, 10mM CaCl 2 , 50mM Tris-HCl, pH 8.0) to elute foreign proteins.
[0070] 4) Add 5CV re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com