Lithium ion battery electrolyte capable of improving high and low temperature cycle performance and lithium ion battery
A lithium-ion battery, high-low temperature cycle technology, applied in the field of lithium-ion battery electrolyte and lithium-ion batteries, can solve the problems of high film-forming resistance and poor low-temperature performance, and achieve low film-forming resistance, high-temperature and low-temperature cycle performance improvement , good effect of battery cycle performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
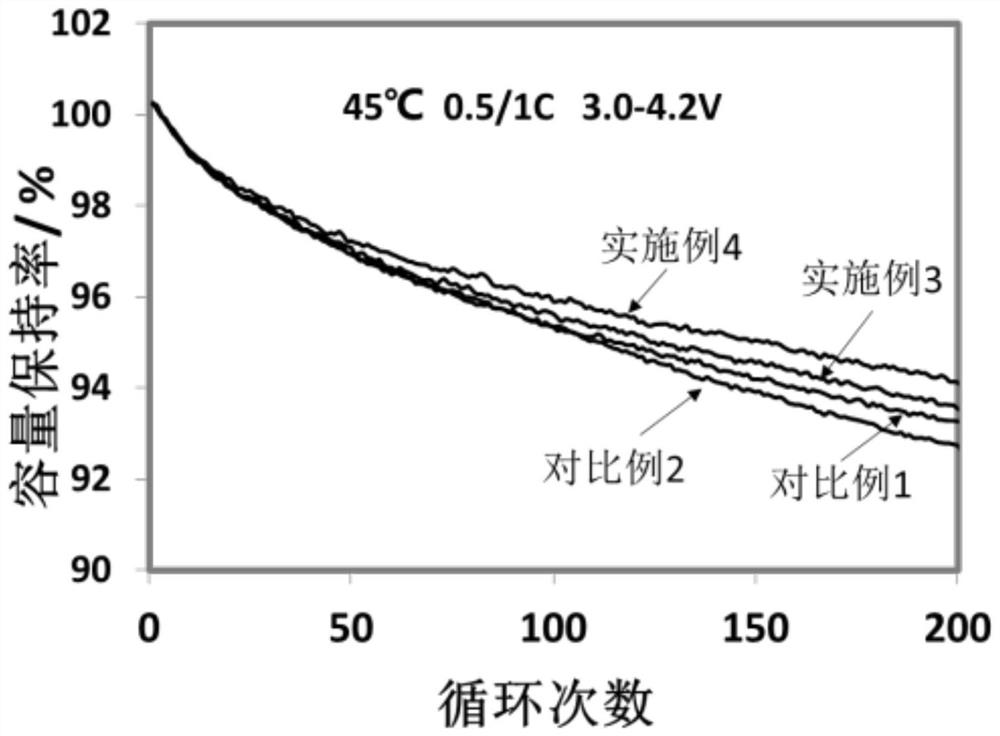
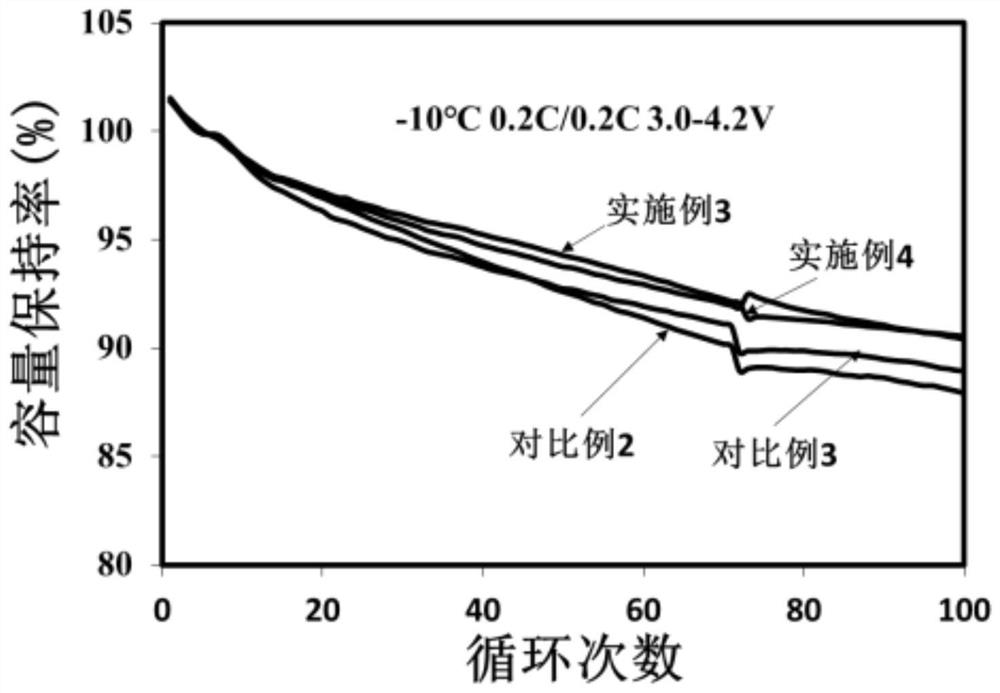
Image
Examples
Embodiment 1
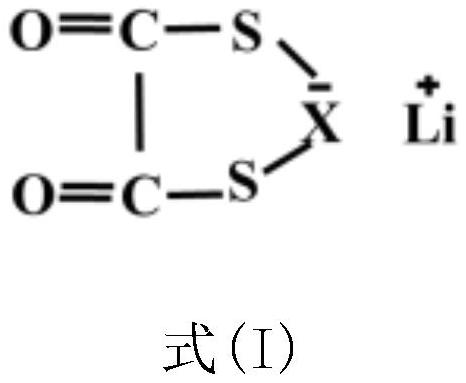
[0030] Preparation of electrolyte additive: lithium difluorodithiooxalate borate, the structural formula is as follows:
[0031]
[0032] Specific steps are as follows:
[0033] (1) In a 2000ml three-necked flask equipped with an electric stirrer and a thermometer, add 500ml of deionized water, control the temperature at 10°C, first add 1mol of dithiooxalic acid, and then slowly add 1mol of lithium carbonate in batches, and fully stir the reaction After filtering to remove insoluble materials, drying under reduced pressure to obtain lithium dithiooxalate with a yield of 92%;
[0034] (2) In a 1000ml three-necked flask equipped with an electric stirrer and a thermometer, add 300ml of acetonitrile, control the temperature at 20°C, first add 2.5mol of lithium dithiooxalate, and then slowly pass in 0.5mol of boron trifluoride in batches. After fully stirring the reaction, the insoluble matter was removed by filtration, and after solvent recrystallization and drying under reduced pressur...
Embodiment 2
[0041] According to the method of Example 1, lithium difluorodithiooxalate borate was prepared.
[0042] Prepare electrolyte 2 and experimental battery 2 according to the method of Example 1. The difference is that in electrolyte 2, the mass percentages of lithium hexafluorophosphate, organic solvent, vinylene carbonate, and lithium difluorodithiooxalate are 13.5% and 75, respectively. %, 1.5%, 10%.
Embodiment 3
[0044] Preparation of electrolyte additive: lithium bisdithio oxalate borate, its structural formula is as follows:
[0045]
[0046] Specific steps are as follows:
[0047] (1) In a 2000ml three-necked flask equipped with an electric stirrer and thermometer, add 500ml of deionized water, control the temperature to 40℃, first add 5mol of dithiooxalic acid, and then slowly add 1mol of lithium hydroxide in batches, and fully stir the reaction After filtering to remove insoluble materials, drying under reduced pressure to obtain lithium dithiooxalate with a yield of 95%;
[0048] (2) In a 1000ml three-necked flask equipped with an electric stirrer and a thermometer, add 300ml of acetone, control the temperature at 60℃, first add 5mol of lithium dithiooxalate, and then slowly pass in 0.5mol of boron trifluoride in batches. After stirring the reaction, the insoluble matter was removed by filtration. After solvent recrystallization and drying under reduced pressure, lithium bisdithiooxala...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com