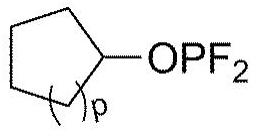
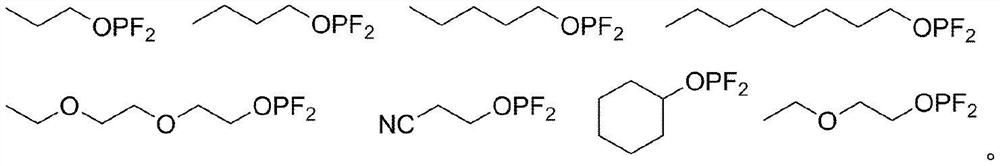
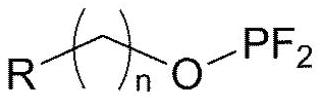Electrolytic solution for lithium secondary battery, and lithium secondary battery comprising same
A secondary battery and electrolyte technology, which is applied in the field of lithium secondary battery and lithium secondary battery electrolyte, to achieve the effects of small thickness change, improved power characteristics, improved cycle characteristics and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] [Example 1] Pentyl difluorophosphite (Pentyldifluorphosphite, CH 3 (CH 2 ) 4 OPF 2 ; hereinafter referred to as "PDFP")
[0109]
[0110] Step 1: Pentyldichlorophosphite (Pentyldichlorophosphite, CH 3 (CH 2 ) 4 OPCl 2 ) preparation
[0111]
[0112] Tetrahydrofuran (200 mL) was added to a 500 mL flask. Phosphorus trichloride (PCl 3 ) (56mL, 0.57mol) and stirred for 10 minutes, then using ice water, the temperature was lowered to a low temperature state of about 0°C. 1-Pentanol (50 g, 0.57 mol) was slowly added dropwise thereto for 30 minutes, then the temperature was raised to normal temperature, and stirred for 3 hours. The reaction mixture was reduced to vacuum to remove volatiles. Analysis was performed using NMR equipment to identify residues and final products. The final product obtained after reduced pressure was pentyl dichlorophosphite (75 g, 0.40 mol) with a purity of almost 100% and a yield of 70%.
[0113] 1 H NMR (500MHz, C 6 D. 6 )δ0.9...
Embodiment 2
[0118] [Example 2] Octyl difluorophosphite (Octyldifluorphosphite, CH 3 (CH 2 ) 7 OPF 2 ; hereinafter referred to as "ODFP")
[0119]
[0120] Step 1: Octyldichlorophosphite (Octyldichlorophosphite, CH 3 (CH 2 ) 7 OPCl 2 ) preparation
[0121]
[0122] Under a nitrogen atmosphere, acetonitrile (15 mL) was added to a 100 mL flask, followed by phosphorus trichloride (PCl 3 ) (5 mL, 57.3 mmol), and the temperature was lowered to -15 °C using brine and dry ice. A solution of 1-octanol (5.97 g, 45.8 mmol) dissolved in acetonitrile (15 mL) was slowly added dropwise thereto for 1 hour, and then the temperature was raised to normal temperature and stirred for 3 hours. The reaction mixture was depressurized to vacuum to remove the solvent, and by further distillation under reduced pressure, octyl dichlorophosphite (6.00 g, 26.0 mmol) was obtained as the final product in a yield of 56.6%.
[0123] 1 H NMR (500MHz, C 6 D. 6 )δ3.85(qui,2H),1.24(m,4H),1.14(m,4H),1.02(m,4...
Embodiment 3
[0128] [Example 3] 2-(2-ethoxyethoxy) ethyl difluorophosphite (2-(2-ethoxyethoxy) ethyldifluorophosphite, CH 3 CH 2 O(CH 2 ) 2 O(CH 2 ) 2 OPF 2 ; hereinafter referred to as "EEEDFP")
[0129]
[0130] Step 1: 2-(2-ethoxyethoxy)ethyl dichlorophosphite (2-(2-ethoxyethoxy)et hyldichlorophosphite, CH 3 CH 2 O(CH 2 ) 2 O(CH 2 ) 2 OPCl 2 ) preparation
[0131]
[0132] Under a nitrogen atmosphere, dichloromethane (38 mL) was added to a 100 mL flask, followed by phosphorus trichloride (PCl 3 ) (5 mL, 57.3 mmol), and the temperature was lowered to 0 °C. 2-(2-Ethoxyethoxy)ethanol (7.54 g, 56.2 mmol) was slowly added dropwise thereto for 1 hour, and then the temperature was raised to normal temperature and stirred for 4 hours. The reaction mixture was depressurized to vacuum to remove the solvent to obtain 2-(2-ethoxyethoxy)ethyl dichlorophosphite (11.08 g, 47.1 mmol) in 83.9% yield as the final product.
[0133] 1 H NMR (500MHz, C 6 D. 6 )δ3.85(qui,2H),3.32(s,4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com