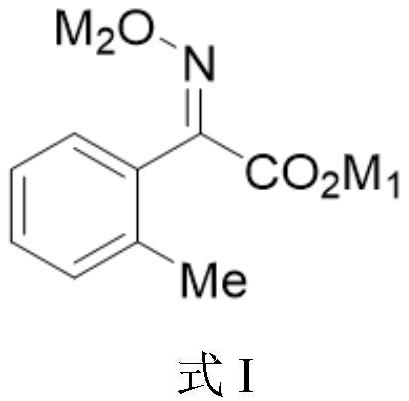
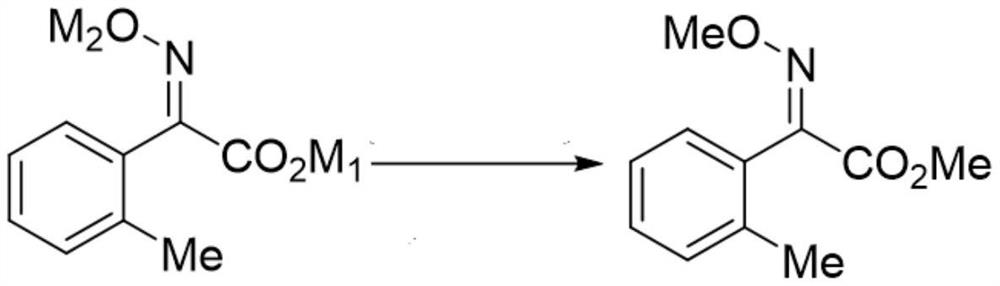
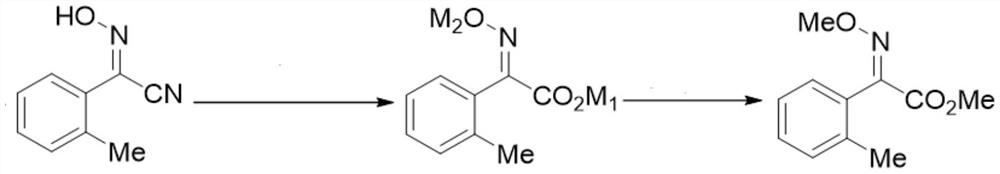
Intermediate for preparing trifloxystrobin and synthesis method of intermediate
A synthetic method, the technology of trifloxystrobin, applied in the field of organic synthesis, can solve the problems of expensive raw materials, serious three wastes, and large amount of three wastes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Embodiment 1 (free acid is used for dimethylation reaction)
[0067] Preparation of 2-oxime-o-tolueneacetonitrile: it is a prior art, such as Example 1 of CN108863845A.
[0068]
[0069] The preparation of (E)-2-oxime-o-methylphenylacetic acid:
[0070] In the reaction flask, add 2-oxime-o-tolueneacetonitrile (64.1 g, 0.4 mol), 36.6 g of sodium hydroxide (0.916 mol), 120 ml of methanol and 100 ml of water, heat to reflux, and intermittently evaporate under reduced pressure Methanol. The internal temperature of the system continued to rise and finally reached about 106°C, which took about 6 hours in total. Cool to room temperature, add 230 ml (0.92 mol) of 4N hydrochloric acid to adjust to pH=1~2, extract 3 times with ethyl acetate, wash with water after combining, dry, and concentrate to obtain 68.3 g of (E)-2-oxime-o-methylbenzene Acetic acid, the liquid phase shows that the trans configuration content is greater than 99%, and the yield is 95%.
[0071] 1 H NMR...
Embodiment 2
[0074] Embodiment 2 (single sodium salt is used for dimethylation reaction)
[0075] Preparation of 2-oxime-o-tolueneacetonitrile: it is a prior art, such as Example 1 of CN108863845A.
[0076]
[0077] The preparation of (E)-2-oxime-o-methylphenylacetic acid monosodium salt:
[0078]
[0079] In the reaction flask, add 2-oxime-o-methylphenylacetonitrile (64.1 g, 0.4 mol), 36.6 g of sodium hydroxide (0.916 mol, 120 ml of methanol and 100 ml of water, heat to reflux, and intermittently distill off methanol The internal temperature of the system continues to rise, and finally reaches about 106 ° C, and takes about 6 hours. Cool to 0 ° C, add 43 ml of concentrated hydrochloric acid (0.51 mol) dropwise under stirring, the reaction solution is directly concentrated, distilled to remove water, and dried to obtain (E)-2-oxime-o-methylphenylacetic acid monosodium salt was directly used in the next methylation reaction without purification, and the purity by HPLC was 99%.
[00...
Embodiment 3
[0084] Embodiment 3 (replace NaOH with KOH, monopotassium salt / chloromethane is used for methylation reaction)
[0085] Preparation of 2-oxime-o-tolueneacetonitrile: it is a prior art, such as Example 1 of CN108863845A.
[0086] (E)-2-oxime-o-methylphenylacetic acid monopotassium salt preparation:
[0087]
[0088] In the reaction flask, add 2-oxime-o-tolueneacetonitrile (64.1 g, 0.4 mol), 51.3 g of potassium hydroxide (0.916 mol), 120 ml of methanol and 300 ml of water, heat to reflux, and intermittently evaporate under reduced pressure Methanol, shared for about 6 hours, the internal temperature of the system continued to rise, and finally reached about 106°C, shared for about 6 hours. Cool to 0°C, add 43ml of concentrated hydrochloric acid (0.51mol) dropwise under stirring, concentrate the reaction solution directly, distill off water, and dry to obtain (E)-2-oxime-o-methylphenylacetic acid monopotassium salt without purification , directly used in the next step of met...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com