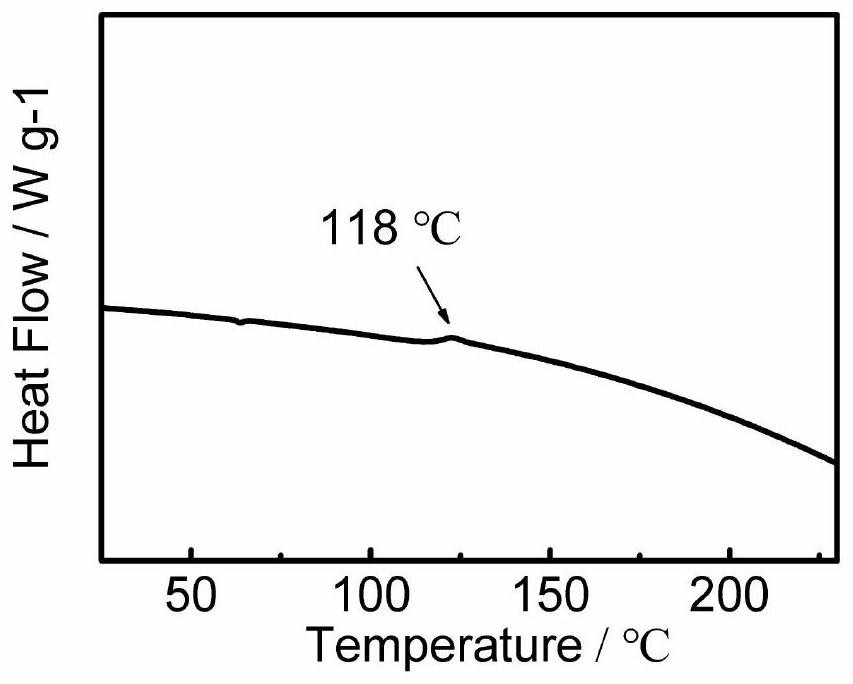
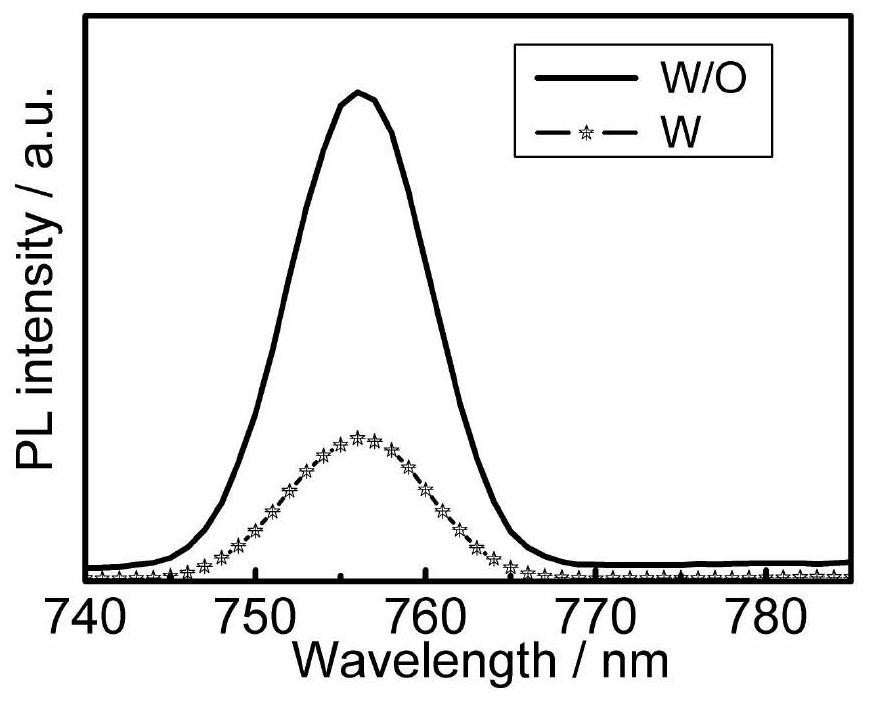
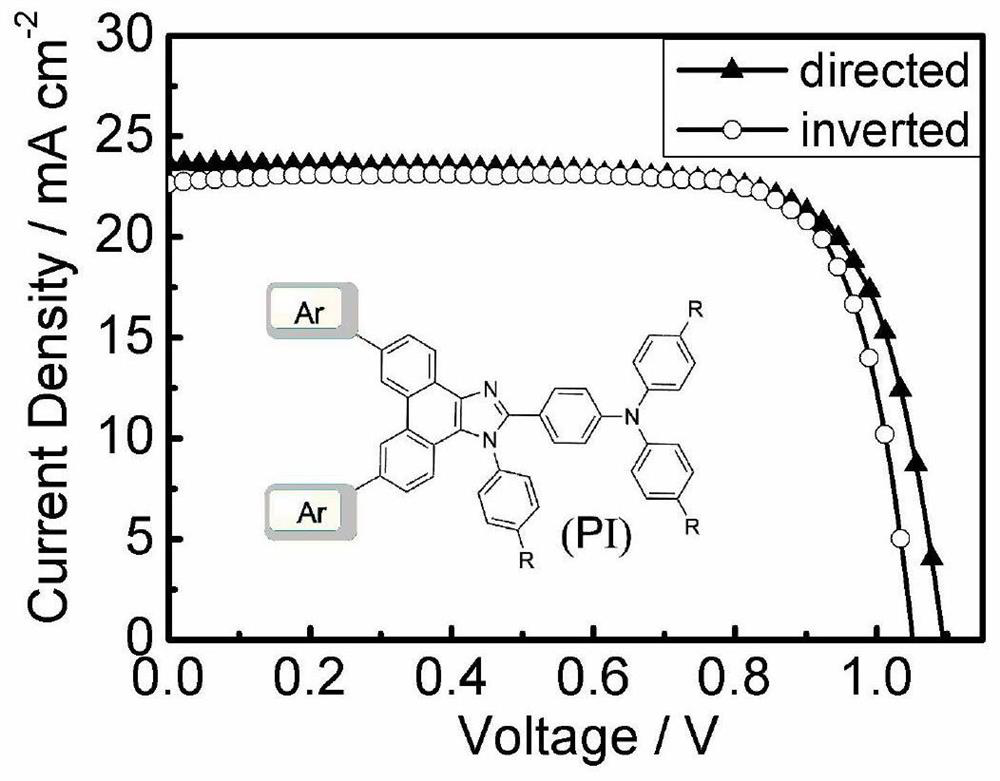
Organic hole transport material taking phenanthroimidazole as parent nucleus and application thereof
A hole transport material, phenanthroimidazole technology, applied in the field of solar cells, can solve the problems of the application of perovskite cells with upright structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Synthetic route of organic hole transport material PI-1 with phenanthrimidazole as the core:
[0039]
[0040] 1) Synthesis of dibromophenanthrimidazole derivative VI
[0041] Add 3,6-dibromophenanthrenequinone (1010mg, 2.74mmol), p-aniline (410mg, 3.29mmol), 4-di-p-methoxyanilinobenzaldehyde (915.55mg, 2.74mmol) and Ammonium acetate (2120mg, 27.48mmol), acetic acid (35ml) as solvent, reflux for 24 hours under nitrogen protection, quench with water, wash with suction, and separate by column chromatography to obtain crude dibromophenanthrimidazole derivative VI , Directly vote the next step.
[0042] 2) Synthesis of organic hole transport material PI-1 with phenanthroimidazole as the core
[0043] 300mg of dibromophenanthrimidazole derivative VI (300mg, 0.51mmol), 4,4’-dimethoxydiphenylamine (350mg, 1.53mmol), Pd 2 (dba) 3 (30mg, 0.06mmol), ligand Xphos (31mg, 0.12mmol) and base t-BuONa (245mg, 2.04mmol), 20mL anhydrous toluene as solvent. The resulting solution was refluxed a...
Embodiment 2
[0047] The synthetic route of the organic hole transport material PI-2 with phenanthrimidazole as the core:
[0048]
[0049] 1) The synthesis of dibromophenanthrimidazole derivative VI is the same as in Example 1
[0050] 2) Synthesis of organic hole transport material PI-2 with phenanthrimidazole as the core
[0051] Add (314mg, 0.4mmol) bromophenanthrimidazole derivative VI, 4-borate-4', 4'-dimethoxytriphenylamine (437mg, 1mmol), Pd(PPh 3 ) 4 (89.2mg, 0.08mmol), add 15ml of ethylene glycol dimethyl ether (DME) as solvent, in N 2 Stir for 5 minutes under atmosphere. Subsequently, 415 mg of potassium carbonate was weighed, and the solution obtained by adding 3 ml of water was added dropwise to the mixture. Continue heating to reflux for 13 hours. It was quenched with water, washed with suction, and finally separated by column chromatography to obtain 321 mg of the organic hole transport material PI-2 with phenanthrimidazole as the core, with a yield of 65.2%.
Embodiment 3
[0053] The synthetic route of the organic hole transport material PI-3 with phenanthrimidazole as the core:
[0054]
[0055] 1) The synthesis of dibromophenanthrimidazole derivative VI is the same as in Example 1
[0056] 2) Synthesis of organic hole transport material PI-3 with phenanthroimidazole as the core
[0057] Add dibromophenanthrimidazole derivative VI (100mg, 0.13mmol), 4,4’-dimethylthiodiphenylamine (100mg, 0.38mmol), Pd to the reaction flask in sequence 2 (dba) 3 (7mg, 0.008mmol), ligand Xphos (8mg, 0.0016mmol) and base t-BuONa (60mg, 0.06mmol), 10mL anhydrous toluene as solvent. The resulting solution was refluxed and stirred for 12 hours under the protection of nitrogen. Cool, add water to quench the reaction, extract, separate and dry the crude product, and distill under reduced pressure to remove the solvent. Finally, 125 mg of the organic hole transport material PI-3 with phenanthrimidazole as the core was obtained by column chromatography, and the yield was 84%....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Open circuit voltage | aaaaa | aaaaa |
| Short circuit current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com