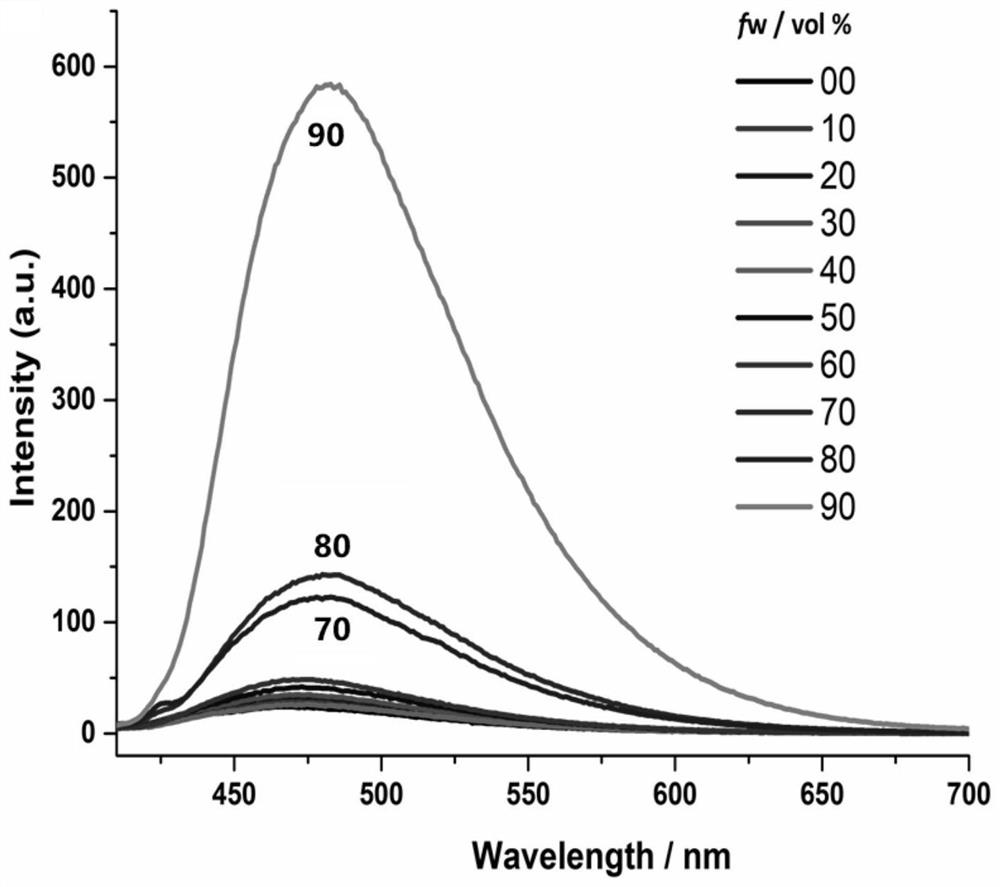
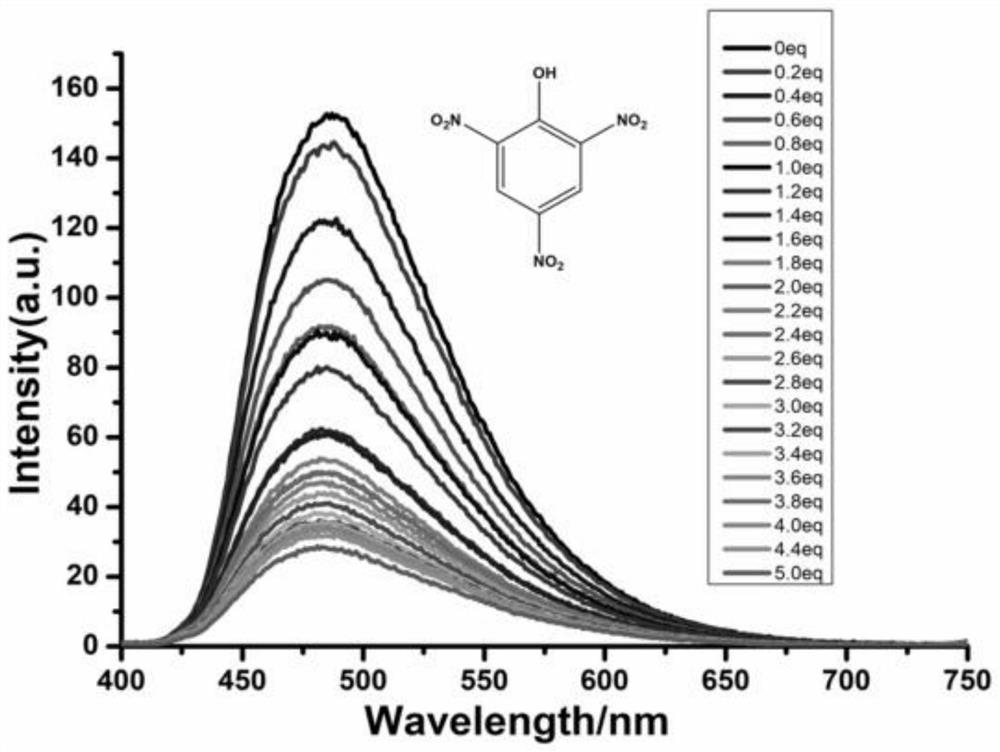
Tetraphenyl ethylene functionalized oligothiophene derivative as well as preparation method and application thereof
A technology of oligothiophene and tetraphenylethylene, applied in chemical instruments and methods, organic chemistry, fluorescence/phosphorescence, etc., can solve problems such as biological toxicity that is difficult to degrade, environmental pollution, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Weigh 2,5-dibromothiophene (32.8mg, 0.14mmol), tetraphenylethyleneboronic acid (113.2mg, 0.308mmol) and cesium carbonate (110.7mg, 0.315mmol) in a 50mL single-necked flask with an analytical balance. Add reflux condenser, stir bar and dropping funnel. Measure 1.5mL of water and 13.5mL of tetrahydrofuran to dissolve the reactants. Finally the catalyst palladium tetraphosphate (16.4 mg, 0.014 mmol) was added. Seal, pump air, heat up to 70 degrees Celsius, continue to stir, condense and reflux for 48 hours. Then the mixture was extracted, extracted with ethyl acetate, dried over anhydrous sodium sulfate, and evaporated in a rotary evaporator to obtain a crude product. The final product—light yellow solid (35.4 mg, 30%) was obtained by eluting with a silica gel column with a mixed eluent of dichloromethane-petroleum ether. FT-IR (KBrpellets, cm -1 ):3424(w), 3075(b), 2423(m), 2852(w), 1598(w), 1492(w), 1443(w), 1074(w), 762(w), 698(m ). 1 HNMR (400MHz, CDCl 3 ):δ7.35...
Embodiment 2
[0036] Weigh 5,5'-dibromo-2,2'-dithiophene (32.4mg, 0.1mmol), tetraphenylethylene boronic acid (82.8mg, 0.22mmol) and cesium carbonate (81.5mg, 0.25mmol) with an analytical balance 50mL single-necked flask. Add reflux condenser, stir bar and dropping funnel. Measure 1.5mL of water and 13.5mL of tetrahydrofuran to dissolve the reactants. Finally the catalyst palladium tetraphosphate (12 mg, 0.01 mmol) was added. Seal, pump air, heat up to 70 degrees Celsius, continue to stir, condense and reflux for 48 hours. Then the mixture was extracted, extracted with ethyl acetate, dried over anhydrous sodium sulfate, and evaporated in a rotary evaporator to obtain a crude product. The final product—light yellow solid (32.2 mg, 35%) was obtained by elution on a silica gel column with dichloromethane-petroleum ether mixed eluent. FT-IR (KBr pellets, cm -1):3426(m),2956(m),2923(m),2852(m),1493(m),1443(m),1073(w),793(m),790(m),695(m ). 1 H NMR (400MHz, CDCl 3 ): δ7.34(s,2H,thiophene),...
Embodiment 3
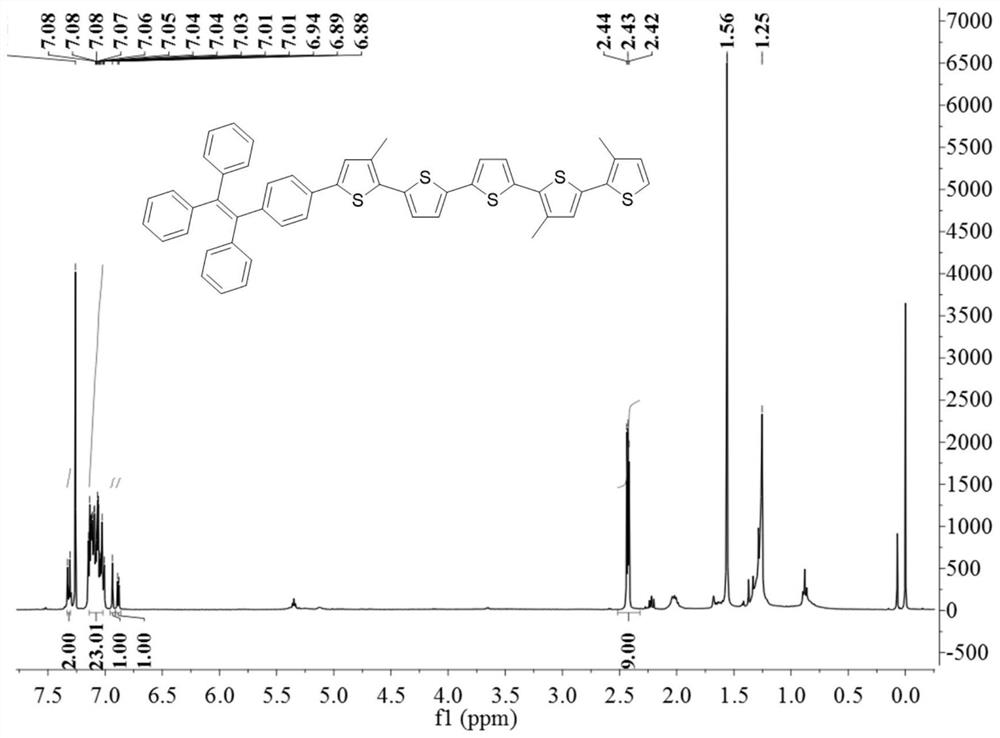
[0038] Weigh 5,5"-dibromo-3,3'-dimethyl-2,2':5',2'-thiophene (43.0mg, 0.1mmol), tetraphenylethyleneboronic acid (83mg, 0.22 mmol) and cesium carbonate (81.5mg, 0.25mmol) in a 50mL single-necked flask. Add a reflux condenser, a stir bar and a dropping funnel. Measure 1mL of water and 9mL of tetrahydrofuran to dissolve the reactant. Finally, add catalyst palladium tetraphosphate (12mg, 0.01 mmol). Sealing, pumping, heating up to 70 degrees Celsius, continuous stirring, condensing and reflux for 48h. Then the mixture was extracted, extracted with ethyl acetate, dried with anhydrous sodium sulfate, and rotated in a rotary evaporator to obtain a crude product. Dichloromethane-petroleum ether mixed eluent, silica gel chromatographic column elution, just obtains final product---yellow solid (20.5mg, 20%).FT-IR (KBr pellets, cm -1 ):3437(m),2956(m),2922(m),2851(m),1597(m),1493(m),1465(m),1442(m),1186(m),1080(m ),968(m),752(m),700(m). 1 H NMR (400MHz, CDCl 3 ):δ7.33(s,2H,thiophene),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com