A kind of pyridazinoquinoxaline diamine Schiff base cobalt ion fluorescent probe and its preparation method
A fluorescent probe, Schiff base cobalt technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of detection effect interference, inability to perform quantitative detection, single detection mode, etc., and achieve good practical value. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
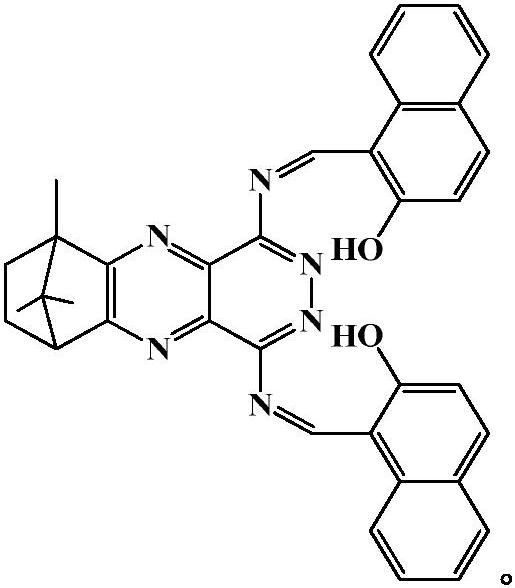
[0030] Preparation of camphorquinone 6,11,11-trimethyl-6,7,8,9-tetrahydro-6,9-metamethylenepyridazino[4,5-b]quinoxaline-1,4- The reaction formula of diamine 2-hydroxy-1-naphthalenecarboxaldehyde bis-Schiff base is:
[0031]
[0032]
[0033] Specific steps are as follows:
[0034] 1) Preparation of 5,9,9-trimethyl-5,6,7,8-tetrahydro-5,8-methylenequinoline-2,3-dicarbonitrile:
[0035] 0.9 mmol of camphorquinone, 1 mmol of 2,3-diaminomaleonitrile and 7 mL of acetic acid were successively added to a dry three-necked flask, the reaction was refluxed for 4 h, followed and monitored by TLC, and the reaction was stopped after the reaction was complete; the reaction solution was decompressed After the acetic acid was distilled off, dichloromethane was added, washed with saturated brine until neutral, and the organic phase was dried over anhydrous sodium sulfate, filtered and concentrated to obtain 5,9,9-trimethyl-5,6,7 , 8-tetrahydro-5,8-methylenequinoline-2,3-dicarbonitrile cru...
Embodiment 2
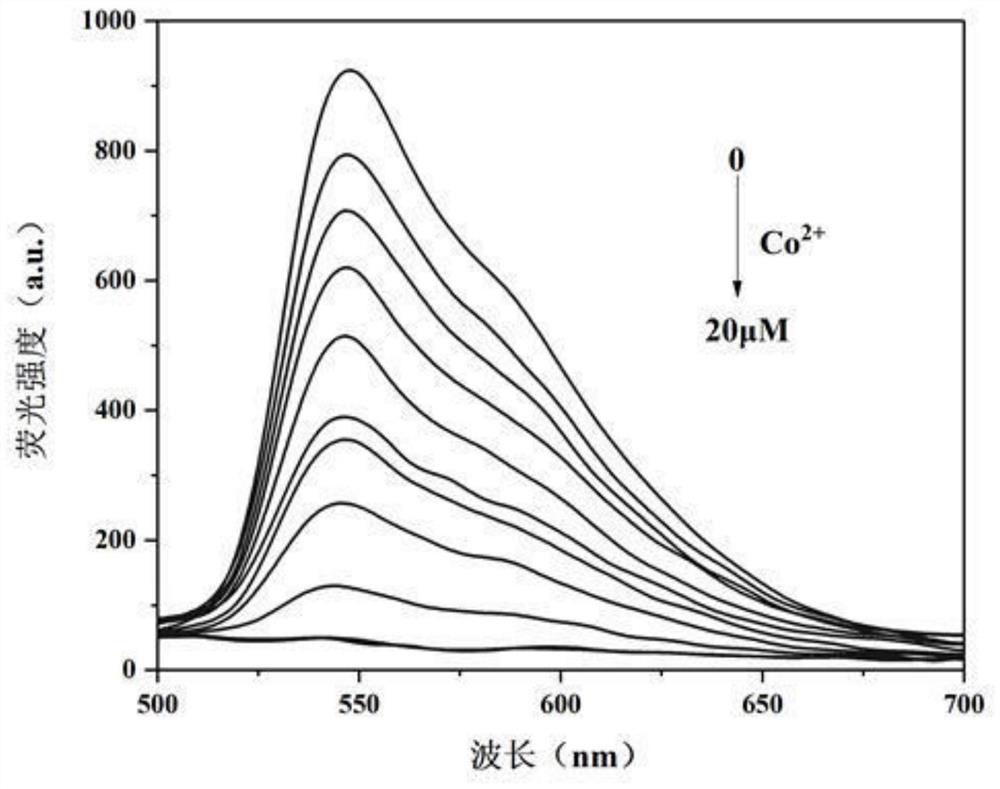
[0041] 6,11,11-trimethyl-6,7,8,9-tetrahydro-6,9-methylenepyridazino[4,5-b]quinoxaline-1,4-diamine 2-Hydroxy-1-naphthaldehyde bis-Schiff base was dissolved in PBS / THF (v / v=6 / 4) buffer to prepare 0.5×10 -5 M concentration probe solution, the CoCl 2 ·6H 2 O was dissolved in PBS / THF (v / v=6 / 4) buffer to prepare solutions with concentrations of 0, 2.0, 4.0, 6.0, 8.0, 10.0, 12.0, 14.0, 16.0, 18.0, 20.0 μM. Different concentrations of cobalt ion pair 6,11,11-trimethyl-6,7,8,9-tetrahydro-6,9-methylenepyridazino were measured by standard titration under spectrofluorophotometer The fluorescence emission spectrum of [4,5-b]quinoxaline-1,4-diamine acetal 2-hydroxy-1-naphthaldehyde bis-Schiff base, as shown in figure 1 shown. The results showed that the orange-yellow fluorescence of the solution was quenched with the increasing concentration of cobalt ions in the system within the concentration range of 0-20 μM, which indicated that the probe could detect cobalt ions sensitively and qua...
Embodiment 3
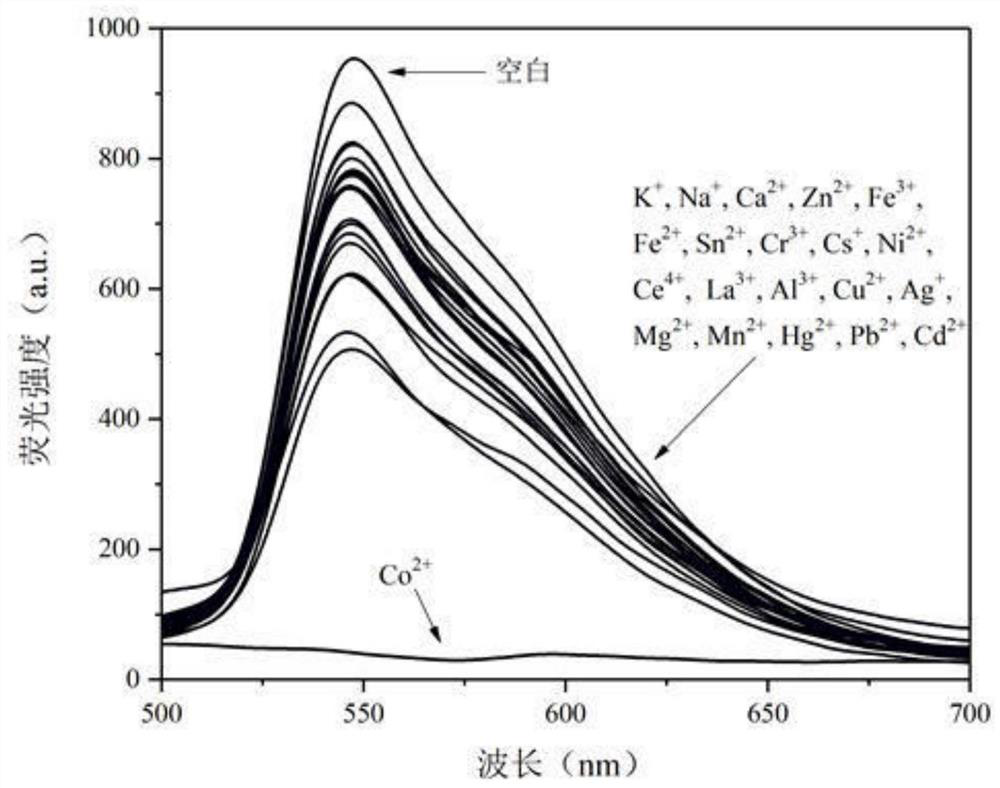
[0043] 6,11,11-trimethyl-6,7,8,9-tetrahydro-6,9-methylenepyridazino[4,5-b]quinoxaline-1,4-diamine 2-Hydroxy-1-naphthaldehyde bis-Schiff base was dissolved in PBS / THF (v / v=6 / 4) buffer to prepare 0.5×10 -5 M concentration of probe solution, various metal salt compounds were dissolved in PBS / THF (v / v=6 / 4) buffer to prepare 1.0×10 -4 M concentration of metal ion solution (Co 2+ , K + , Na + , Ca 2+ , Zn 2+ , Fe 3+ , Fe 2+ , Sn 2+ , Cr 2+ , Cs + , Ni 2+ , Ce 4+ , La 3+ , Al 3+ , Cu 2 + , Ag + , Mg 2+ , Mn 2+ , Hg 2+ , Pb 2+ , Cd 2+ ). Different ion pairs 6,11,11-trimethyl-6,7,8,9-tetrahydro-6,9-methylenepyridazino[4 , 5-b]quinoxaline-1,4-diamine acetal 2-hydroxy-1-naphthaldehyde bis-Schiff base fluorescence emission spectrum, such as figure 2 shown. The results show that the fluorescence is significantly quenched after the addition of cobalt ions to the probe solution, while the addition of other ions such as K + , Na + , Ca 2+ , Zn 2+ , Fe 3+ , Fe 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com