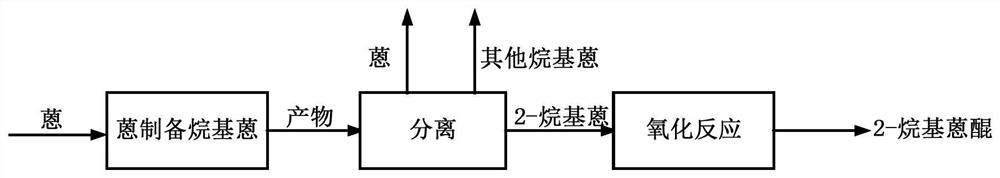
Method for separating 2-alkyl anthracene from anthracene through reaction and preparing 2-alkyl anthraquinone through catalytic oxidation
An alkyl anthracene, separation technology, applied in the field of preparation of organic matter
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
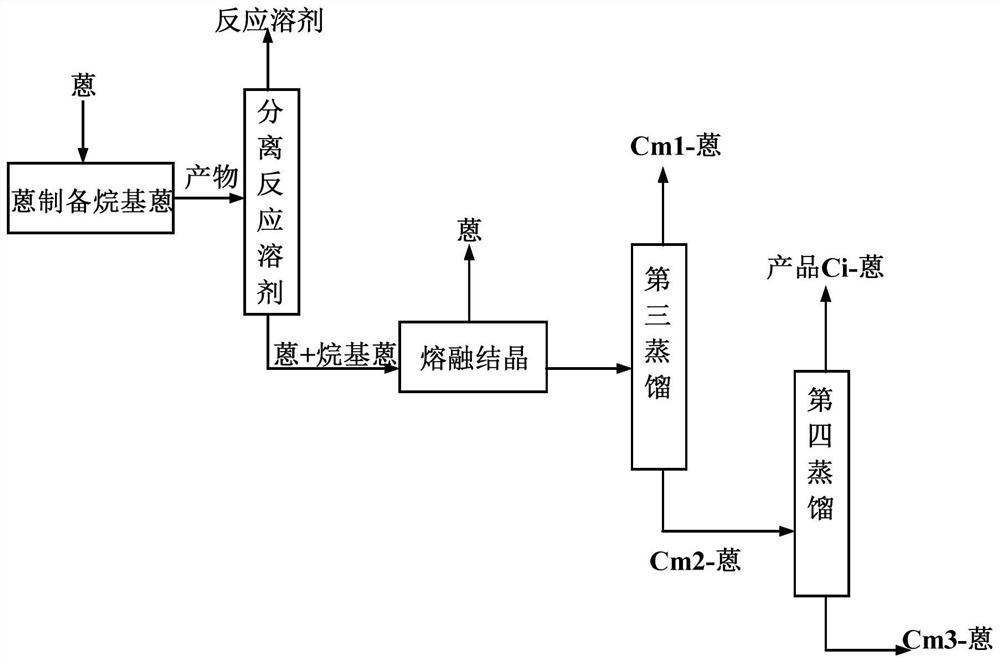
[0055] According to a specific embodiment of the present invention, in step (2-3), the method for described multi-step distillation comprises:
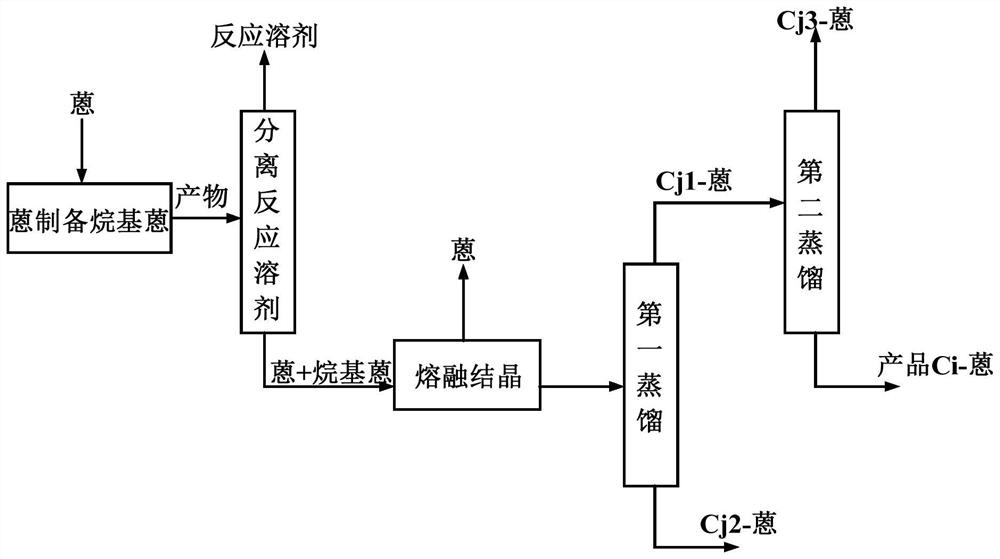
[0056] Method 1: if figure 2 As shown, the feed liquid of a series of alkylanthracene products containing 2-alkylanthracene is subjected to the first distillation, and the distillate containing the light component Cj1-anthracene and the bottom product containing the heavy component Cj2-anthracene are separated; The distillate containing the light component C1j-anthracene is subjected to a second distillation to obtain a distillate containing the light component Cj3-anthracene, and a bottom product containing the target product Ci-anthracene;
[0057] Among them, the light component Cj1-anthracene is an alkyl anthracene product whose total carbon number j1 of the alkyl side chain is an integer of 1<j1<i+1, and the heavy component Cj2-anthracene is the total carbon number of the alkyl side chain j2 is i< The alkylanthracene product of...
Embodiment 1
[0112] (1) Alkylation reaction.
[0113] Alkylation of anthracene and isopentene to prepare 2-pentylanthracene, mesitylene as solvent and methanesulfonic acid as catalyst. At room temperature, 460 g of anthracene, 800 ml of mesitylene, and 42 g of methanesulfonic acid were added to a 2L stirred tank. After sealing, the temperature is raised to 165° C. at a rotational speed of 1000 rpm, and the pressure is 0.3 MPa. 151 g of isoamylene was added to the kettle through a plunger pump, and the feed rate was 6.6 g / min. After the feeding of isopentene was completed, the reaction was continued for 270 min while maintaining the reaction conditions, and then the reaction was terminated. 10 batches were reacted under the same conditions. After the catalyst was separated, the alkylation reaction product was collected uniformly as the raw material for the separation of alkylanthracene.
[0114] (2) Separation.
[0115] The alkylation reaction product is sent to the atmospheric distilla...
Embodiment 2
[0125] Prepare 2-alkylanthraquinones according to the method of Example 1, the difference is that in step (2), the cooling rate is 5.0°C / h, the crystallization temperature is 190°C, and the amount of seed crystal anthracene added accounts for 4% of the mass of the molten mixture. % by weight, the crystal growth time is controlled at 4h. After the crystallization process is over, the uncrystallized feed liquid is discharged and sent to the vacuum distillation system. Slowly heat up and sweat the crystal in the crystallizer, the heating rate is 4°C / h, the sweating end temperature is 195°C, the sweating amount is 10% by weight of the crystal mass, the sweating liquid circulates and contacts the material entering the melting crystallizer After that, the crystallization operation is carried out together. The uncrystallized alkylanthracene mixture is sent to the vacuum distillation system for the first vacuum distillation. The top pressure is 1KPa, the bottom temperature is 300°C, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com