Agonist of deacetylase and application thereof
A deacetylase and agonist technology, applied in the field of medicine, can solve the problems of not studying the protein targets of cinnamic aldehyde structural analogs and cinnamaldehyde derivatives, and the lack of in-depth research on the protein targets of cinnamaldehyde.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] This example uses the Target Hunter software to predict the binding of cinnamic aldehyde to the target protein in vivo
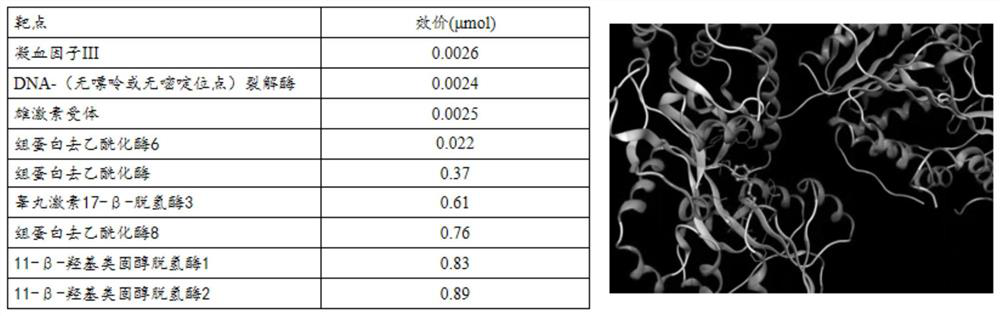
[0041] Prediction results: if figure 1 As shown, according to the 3D structure of the small molecule of cinnamon aldehyde, the Target Hunter software combines CVDPlatform to predict the protein target of cinnamic aldehyde binding in vivo, and selects the top 5 proteins according to the p-value, among which 3 prediction results show that cinnamon aldehyde and histone deacetylation There is a strong binding effect on deacetylase 6, 8 or histone deacetylase.
Embodiment 2
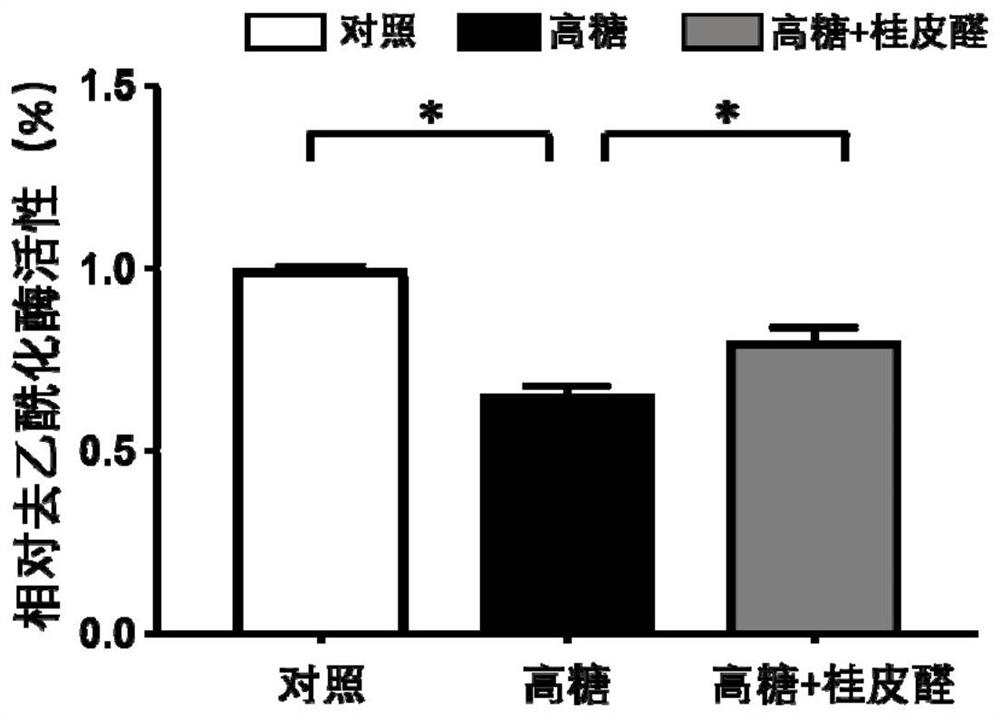
[0043] This example is a study of the effect of cinnamaldehyde on the HDAC activity of endothelial cells induced by high glucose
[0044] 1. Cell culture
[0045] Inoculate HUVEC in human umbilical vein endothelial cell culture medium containing 10% fetal bovine serum and 100 U / ml double antibody, and place at 37°C, 5% CO 2 Culture in an incubator, digest and subculture when the cells are cultured to 80%-90%.
[0046] 2. Drugs and main reagents
[0047] Cinnamaldehyde (Trans-cinnamaldehyde, TCA) was purchased from Sigma-Aldrich Company (China, Shanghai); Dimethylsulfoxide (DMSO) was purchased from Sigma-Aldrich Company (China, Shanghai); glucose (D(+)- Glucose), purchased from Sigma-Aldrich Company (China, Shanghai); human umbilical vein endothelial medium, purchased from Beijing Yuhengfeng Company (China, Beijing).
[0048] 3. Experimental method
[0049] 1. Cell grouping and high-glucose modeling: the cells were divided into 3 groups, normal control group (control), high...
Embodiment 3
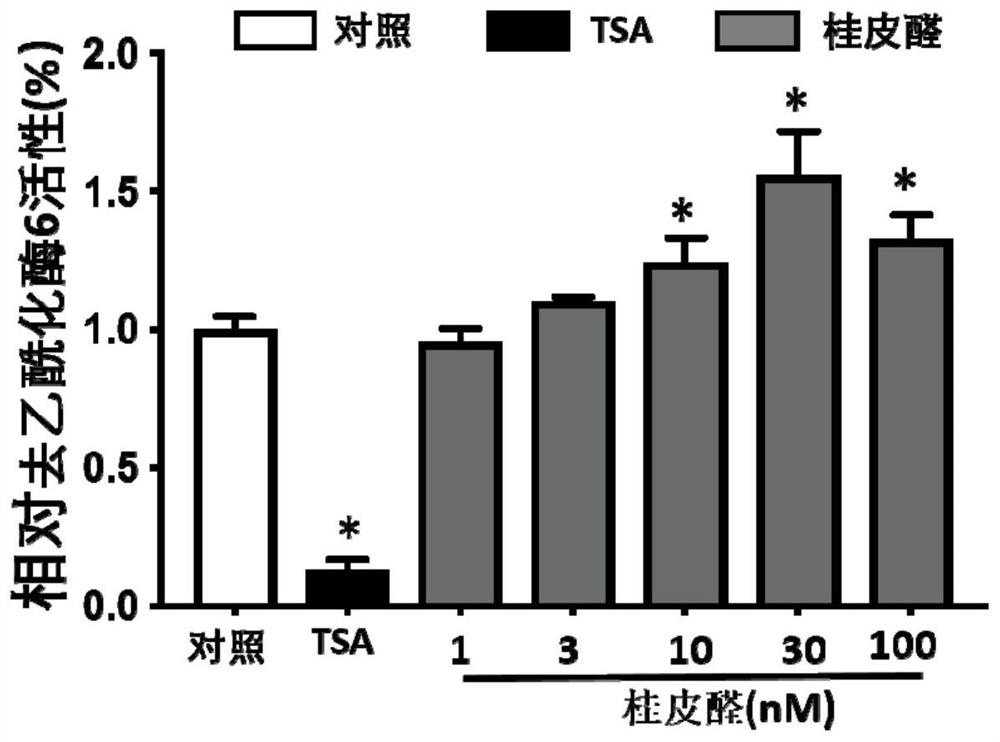
[0059] This example is a study of the agonistic effect of cinnamic aldehyde on HDAC6 activity
[0060] One, cell culture: with embodiment 2.
[0061] Two, medicines and main reagents: with embodiment 2.
[0062] 3. Experimental method
[0063] HDAC6 activity kit detection (Enzo Life Sciences, BML-AK516): the kit uses the FluordeLys system (fluorescent histone deacetylase lysine substrate / developer), which can be used for specific histone deacetylase 6 detection . The assay process is divided into two steps, first, the SIRT1 substrate containing acetylated lysine side chain is incubated with HDAC6, the deacetylation of the substrate will make the substrate sensitive, in the second step, it is treated with the developer Fluorophores are produced. The operation steps are as follows:
[0064] 1) Make cinnamon aldehyde into different final concentrations according to the gradient: 10 μ TCA (diluted with DMSO, diluted with Buffer in the last step) is added to 140 μ mixed soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com