Application of calcium ion carrier in leucoderma treatment
A calcium ion carrier and vitiligo technology, applied in the field of medicine, can solve the problems of melanocytes' disturbance of calcium ion uptake and transport, and the inability to effectively correct vitiligo drugs and methods.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
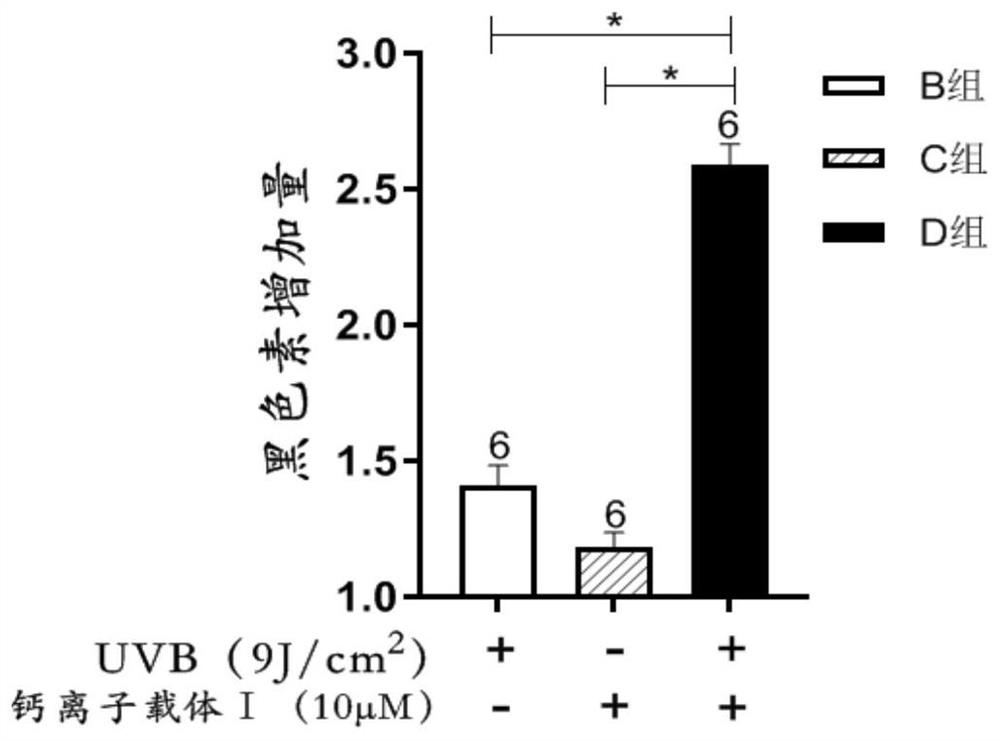
[0030] Combined use of calcium ionophore Ⅰ and narrow-wave UVB in the treatment of vitiligo, and observe its effect on the synthesis of melanin in human melanocytes.
[0031] 1. Materials
[0032] 1.1 Cell line: Melanocytes around human vitiligo lesions, donated by the Dermatology Department of the First Affiliated Hospital of Kunming Medical University.
[0033] 1.2 Drug: calcium ionophore Ⅰ, CAS No.58801-34-6, specification: 50mg, brand: Sigma.
[0034] 1.3 Reagents and consumables: Melanocyte culture medium (Melm) was purchased from American Blue and White Company, fetal bovine serum was purchased from German PAN Company, trypsin was purchased from Shanghai Sangon Bioengineering Co., Ltd., dimethyl sulfoxide (DMSO) was purchased from The 3.5 cm cell culture plate was purchased from CORNING Company of the United States, the standard melanin was purchased from Sigma Company of the United States, and the BCA protein concentration determination kit was purchased from Beyontian...
Embodiment 2
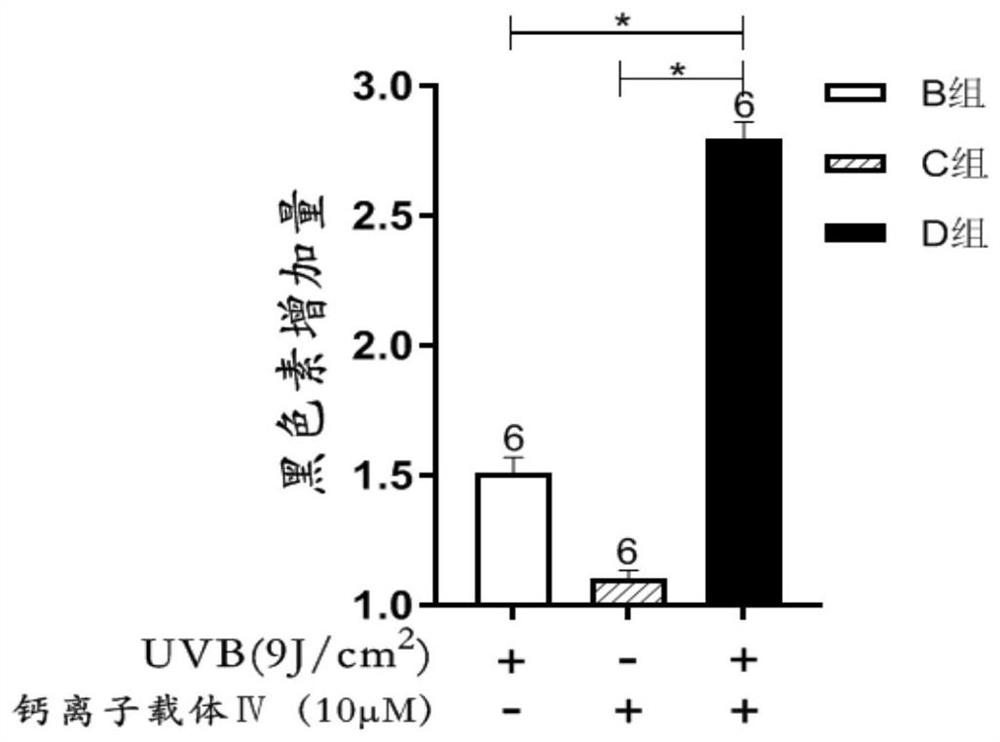
[0044] Combined use of calcium ionophore Ⅳ and narrow-wave UVB to observe its effect on the synthesis of melanin in melanocytes. Except following difference, other is with embodiment 1.
[0045] 1 material
[0046] 1.2 Drug: calcium ionophore IV, CASNo.126572-74-5, specification: 50mg, brand: Sigma.
[0047] 2 test method
[0048] 2.2 Experimental grouping: The experiment was divided into 4 groups, namely negative control group (group A), UVB single action group (B group), calcium ionophore Ⅳ single action group (C group), UVB and calcium ionophore Ⅳ joint action group (Group D), the variables in the above 4 groups were consistent except for the relevant variables. In the negative control group, the cells in this group were not treated; in the UVB alone action group, the cells in this group were irradiated with a vitiligo UVB phototherapy instrument, and the irradiation dose was 9J / cm 2 , and then continue to culture for 12 hours; calcium ionophore Ⅳ alone action group, ad...
Embodiment 3
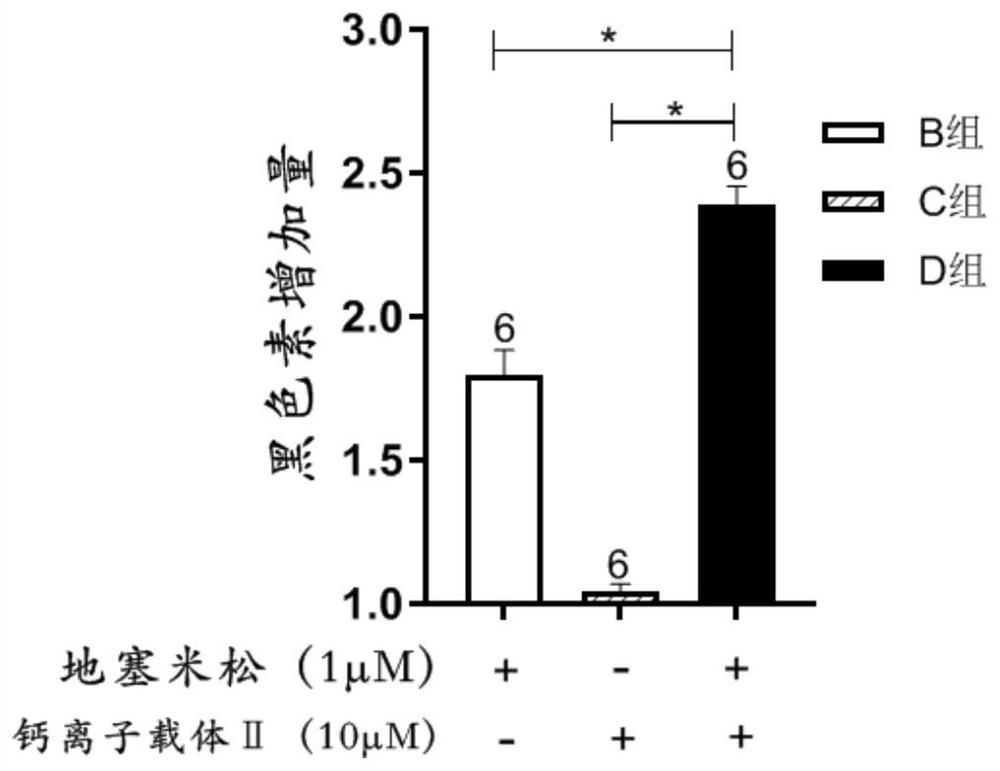
[0052] Combined use of calcium ionophore Ⅱ and dexamethasone to observe its effect on melanin synthesis by melanocytes. Except following difference, other is with embodiment 1.
[0053] 1 material
[0054] 1.2 Drug: calcium ionophore II, CAS No.74267-27-9, specification: 50mg, brand: Sigma. Dexamethasone, CAS No.50-02-2, specification: 100mg, brand: Sigma.
[0055] 2 Experimental methods
[0056] 2.2 Experimental grouping: The experiment was divided into 4 groups, namely negative control group (group A), dexamethasone alone action group (group B), calcium ionophore II alone action group (group C), dexamethasone and calcium ionophore In the Ⅱ joint action group (group D), the variables in the above 4 groups were consistent except for the relevant variables. In the negative control group, this group of cells was not treated; in the dexamethasone alone group, dexamethasone was added to the cell culture medium of this group to make the concentration of 1 μmol / L, and then conti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com