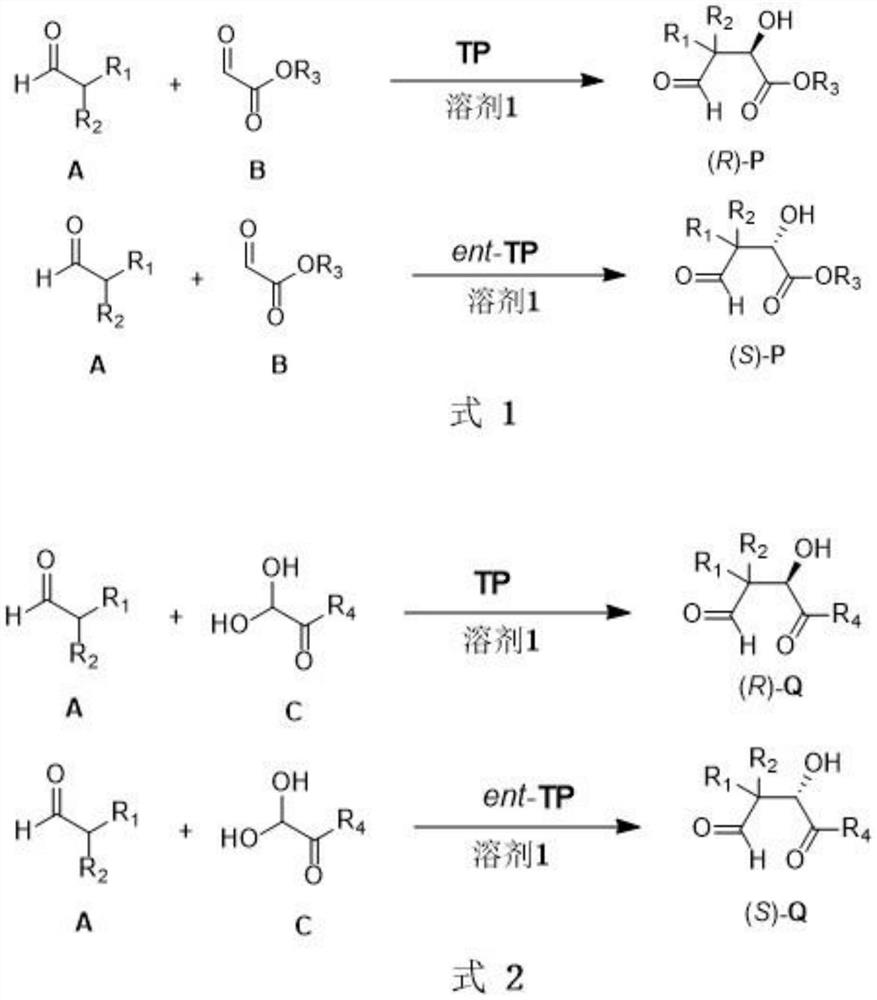
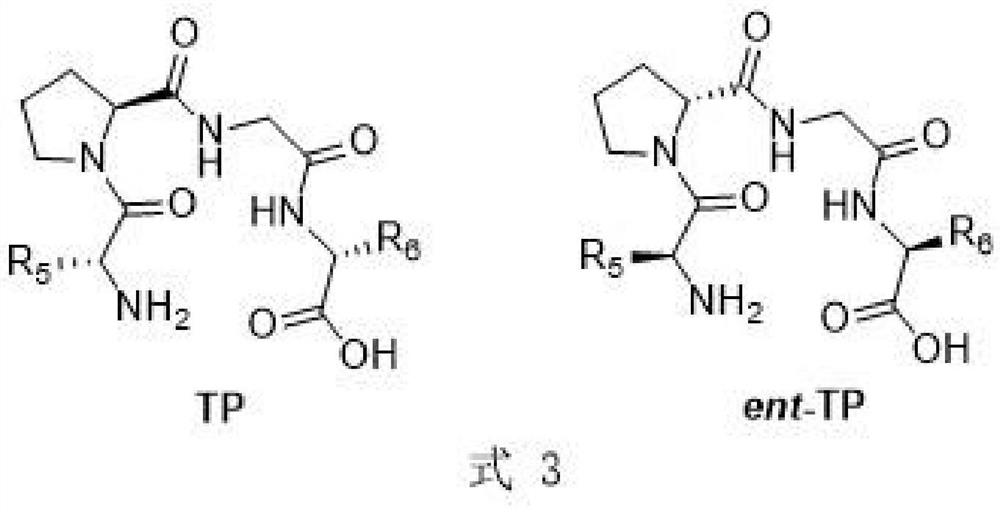
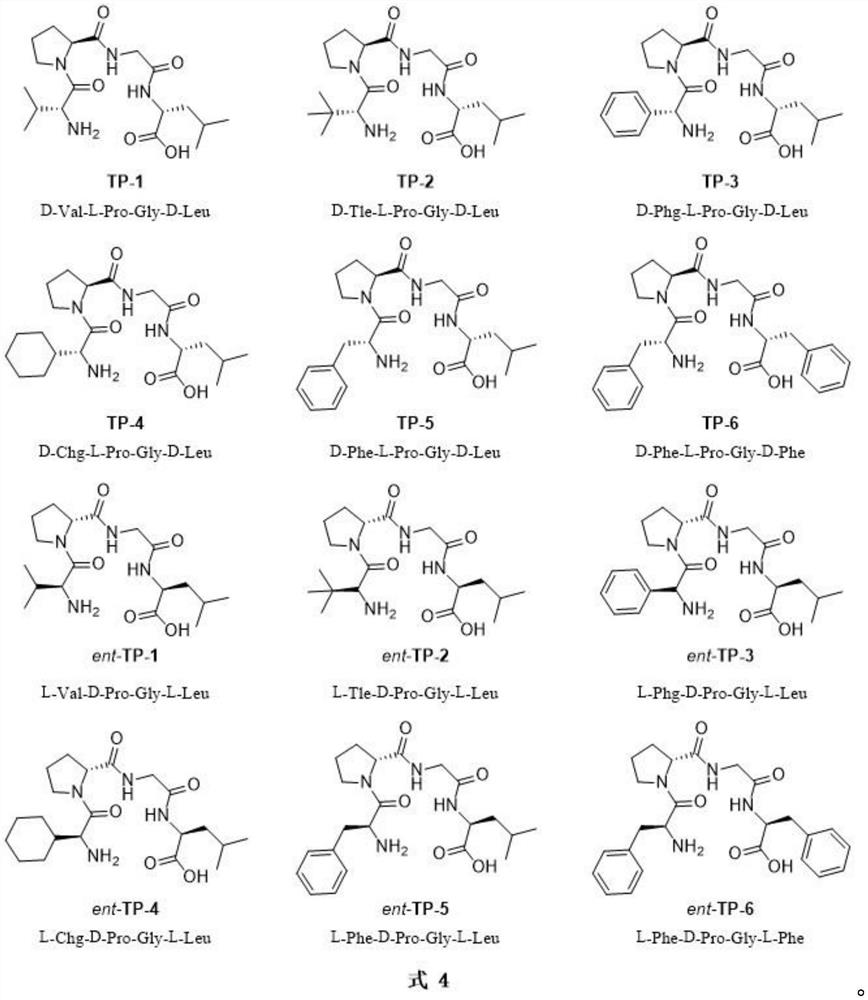
Synthesis method of chiral 2-hydroxy-1, 4-dicarbonyl compound and pantoic acid lactone
A kind of pantolactone, monohydrate technology, applied in the field of synthetic products, can solve problems such as no systematic report
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023]
[0024] Add accurately weighed tetrapeptide catalyst ent-TP (0.025mmol) and 1.0mL dichloromethane into a 5mL round bottom flask, add isobutyraldehyde (92μL, 1.0mmol) while stirring in an ice-water bath, and then add glyoxylic acid Ethyl ester (50% toluene solution, 0.1 mL, 0.5 mmol), the reaction was returned to room temperature and stirred, detected by TLC, and the progress of the reaction was judged by color development with 2,4-dinitrophenylhydrazine. After the reaction, add 3-4 drops of saturated ammonium chloride solution, extract with ethyl acetate (10mL×3), wash with a small amount of saturated brine, dry over anhydrous sodium sulfate, concentrate under reduced pressure, and purify the residual oil by column chromatography. The product (S)-P-1 is obtained.
[0025] Table 1. The results of tetrapeptide catalysts ent-TP-1~ent-TP-6 catalyzing the asymmetric Aldol reaction between isobutyraldehyde and ethyl glyoxylate
[0026]
[0027] a Isolated yield. b The...
Embodiment 2
[0029]
[0030] Add accurately weighed tetrapeptide catalyst ent-TP-2 (0.025mmol, 10mg) and 1.0mL solvent into a 5mL round bottom flask, add isobutyraldehyde (92μL, 1.0mmol) while stirring in an ice-water bath, and then add ethyl Ethyl alkydate (50% toluene solution, 0.1 mL, 0.5 mmol), the reaction was returned to room temperature and stirred, detected by TLC, and the progress of the reaction was judged by color development with 2,4-dinitrophenylhydrazine. After the reaction, add 3-4 drops of saturated ammonium chloride solution to quench the reaction, extract with ethyl acetate (10mL×3), wash with a small amount of saturated brine, dry over anhydrous sodium sulfate, concentrate under reduced pressure, and purify the residue by column chromatography. As an oil, (S)-P-1 was obtained.
[0031] Table 3. Effect of solvent on the asymmetric Aldol reaction of isobutyraldehyde and ethyl glyoxylate catalyzed by tetrapeptide ent-TP-2
[0032]
[0033] a Isolated yield. b The e...
Embodiment 3
[0035]
[0036] Add accurately weighed tetrapeptide catalyst TP (0.025mmol) and 1.0mL acetonitrile into a 5mL round bottom flask, add isobutyraldehyde (92μL, 1.0mmol) while stirring in an ice-water bath, and then add ethyl glyoxylate (50 % toluene solution, 0.1mL, 0.5mmol), the reaction was returned to room temperature and stirred, detected by TLC, and the progress of the reaction was judged by color development with 2,4-dinitrophenylhydrazine. After the reaction, add 3-4 drops of saturated ammonium chloride solution, extract with ethyl acetate (10mL×3), wash with a small amount of saturated brine, dry over anhydrous sodium sulfate, concentrate under reduced pressure, and purify the residual oil by column chromatography. The reaction product (R)-P-1 was obtained.
[0037] Table 2. Tetrapeptides TP-1~TP-6 catalyze the asymmetric Aldol reaction of isobutyraldehyde and ethyl glyoxylate
[0038]
[0039] a Isolated yield. b The ee value was determined from the benzoate es...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com