Preparation method of benzofuran derivative
A technology of benzofuran and derivatives, applied in the field of organic chemical synthesis, can solve the problems of complex reaction system, high reaction temperature, metal ion waste water and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1: Preparation of o-hydroxystilbene and its derivatives
[0055] O-hydroxystilbene and its derivatives can be purchased from the market or prepared. In this example, o-hydroxystilbene and its derivatives were synthesized by using the McMurry method. The reaction equation is as follows:
[0056]
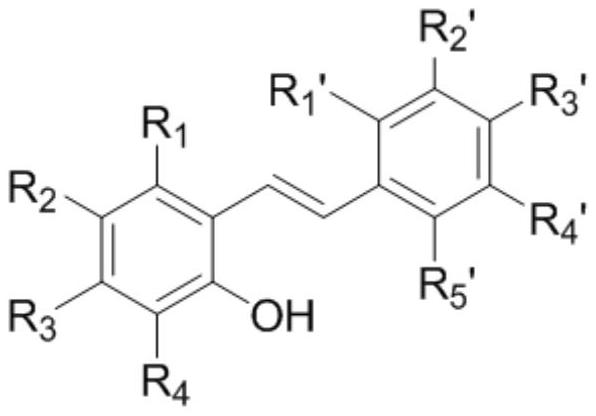
[0057] Among them, R 1 , R 2 , R 3 , R 4 , R 1 ’, R 2 ’, R 3 ’, R 4 ’, R 5 'is an aryl group, an acid (amine) group, a carboxylic acid (ester) group, a sulfonic acid (amine, ester) group, a sulfinic acid (amine, ester) group, a nitrile group, a (cyclo) alkyl group, an alkenyl group, an alkyne group Any one of halogen, alkoxy, amino, amino, thioether, nitro, silicon, and phosphonic acid (ester) groups.
[0058] The specific preparation method is as follows: adding zinc powder and a stirring bar into a pre-dried two-neck flask, sealing and degassing, and adding anhydrous tetrahydrofuran under the protection of argon. The solution was cooled to -5°C, at whic...
Embodiment 2
[0061] Example 2: Preparation of a 2-arylbenzofuran compound
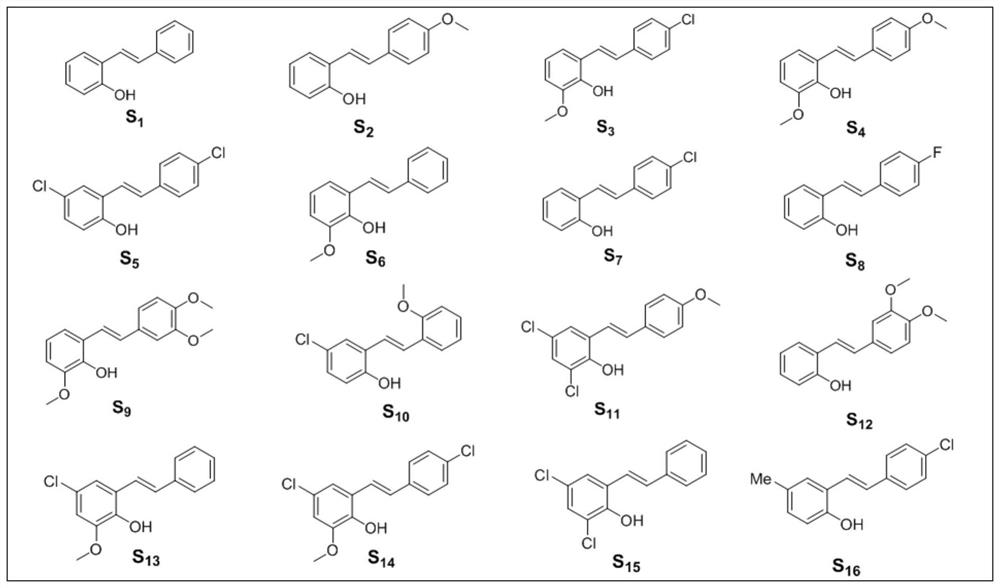
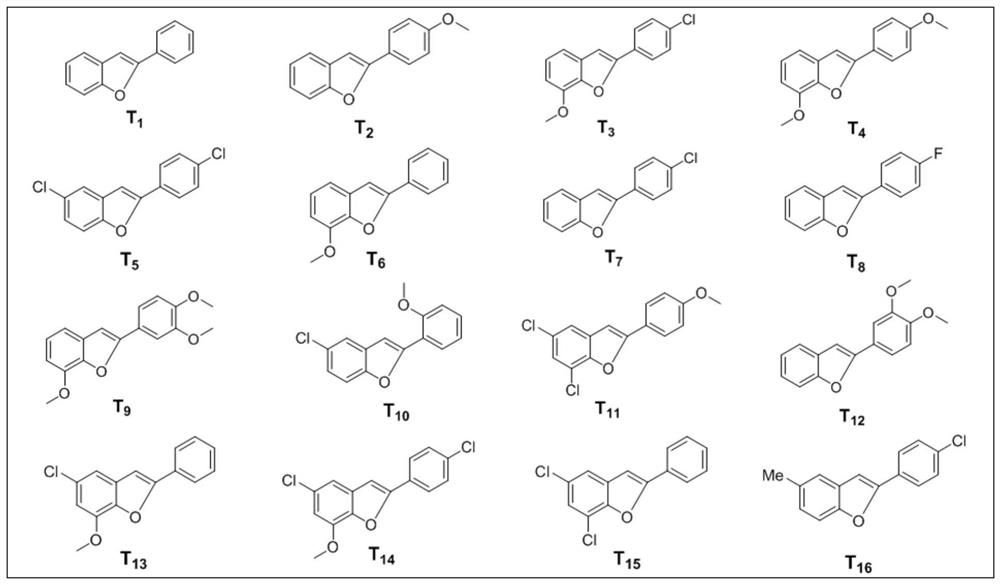
[0062] In a 15mL single-port reaction tube, add 10mg (0.05mmol) (E)-2-styrylphenol (structure as figure 1 S in 1 shown) and 24 μL (0.1 mol) of dodecylmercaptan, stirred and reacted at 140° C. for 48 h under an oxygen atmosphere. Add 1 mL of water to quench the reaction, extract with ethyl acetate (3 times×2 mL / time), and combine the organic phases. After drying the organic phase with anhydrous sodium sulfate, the organic phase was distilled off under reduced pressure to remove the solvent, and the residue was separated through a 200-300 mesh silica gel column, and the eluent was PE:EA=10:1 (v / v, implemented later) Examples are all volume ratios, no more details), to obtain the target product, the structure is as figure 2 T in 1 shown. Obtain the target product T 1 6.5 mg, yield 65%. The reaction formula is as follows:
[0063]
[0064] Target product T 1 It is a white solid, and its spectral data are a...
Embodiment 3
[0066] Example 3: Preparation of another 2-arylbenzofuran compound
[0067] In a 15mL single-port reaction tube, add 10mg (0.044mmol) of (E)-2-(4-methoxystyryl)phenol (structure as figure 1 S in 2 shown) and 21 μL (0.088 mmol) of dodecylmercaptan, stirred at 140° C. for 48 h under an oxygen atmosphere. Add 1 mL of water to quench the reaction, extract with ethyl acetate (3 times × 2 mL / time), combine the organic phases, dry the organic phases with anhydrous sodium sulfate, distill off the solvent under reduced pressure, and separate the residue through a 200-300 mesh silica gel column , the eluent is PE:EA=10:1, the target product is obtained, the structure is as figure 2 T in 2 shown. Obtain the target product T 2 4.7 mg, yield 47%. The reaction formula is as follows:
[0068]
[0069] Target product T 2 It is a white solid, and its spectral data are as follows:
[0070] 1 H NMR (600MHz, CDCl 3 ): δ7.80(d, J=8.8Hz, 2H), 7.55(d, J=7.5Hz, 1H), 7.50(d, J=8.1Hz, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com