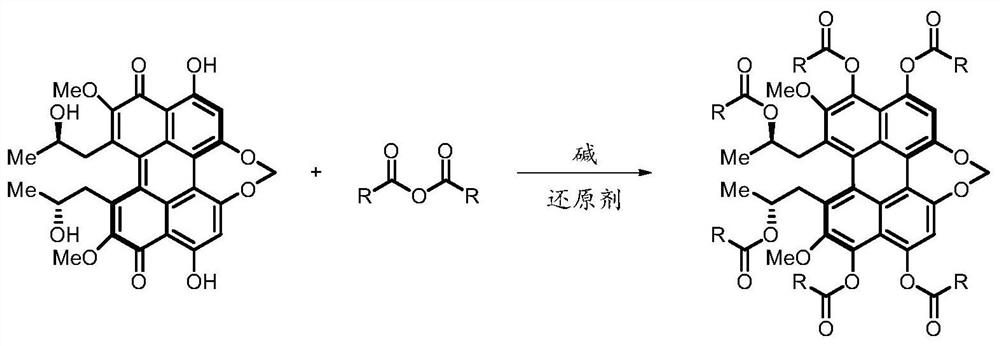
Hexaacyl reductive cercosporin photocatalyst, and preparation method and application thereof
A methyl and ethyl technology, applied in the field of catalyst preparation and organic synthesis, can solve the problem of low photoactivity of cercosporine, and achieve the effect of low cost, simple method and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
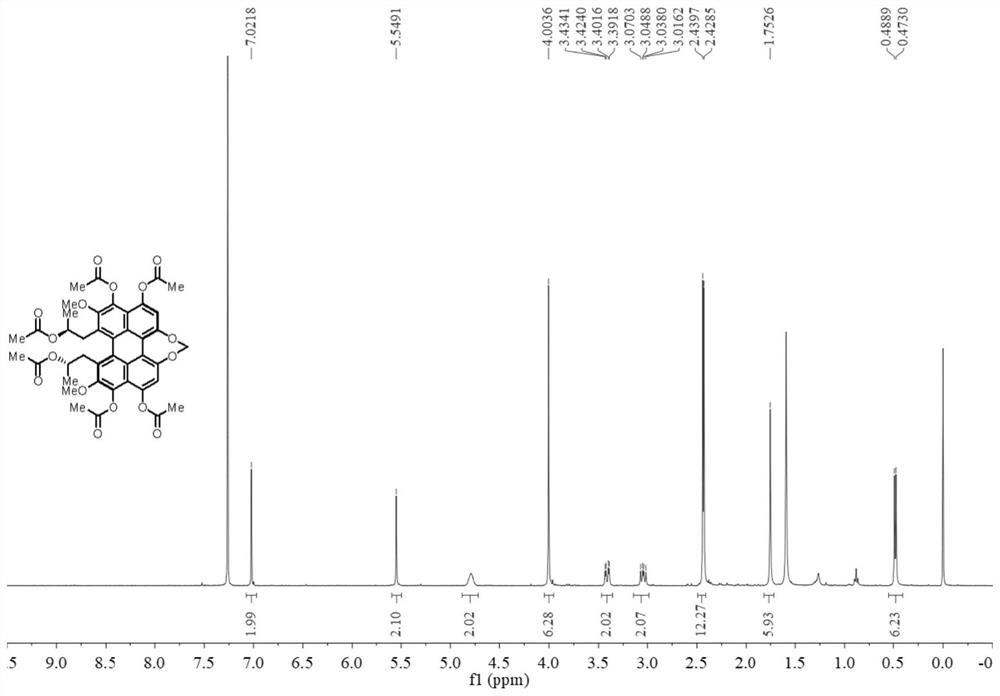
[0046] [Example 1] Preparation of Hexaacetyl Reductive Cercosporine
[0047]
[0048] The reaction formula of this example is as shown above, and the specific reaction method is: cercosporin (20 mg), acetic anhydride (400 μL), magnesium powder (30 mg), N,N-dimethyl-4-aminopyridine ( 50 mg) and a stirring bar were added into the reaction vessel, and reacted for 2 hours under nitrogen protection. After the reaction was completed, acetic anhydride (400 μL) was added again, and the reaction was continued for 2 hours. After the reaction was completed, 20 mL of dichloromethane was added to the reaction liquid, and then filtered, and the obtained filtrate was washed three times with 10 mL of distilled water. Finally, the organic phase was dried by adding anhydrous magnesium sulfate, and the filtrate was obtained by suction filtration, and concentrated using a rotary evaporator. Quickly separate with 200-300 mesh silica gel column chromatography, the eluent used is methanol / dichl...
Embodiment 2
[0051] [Example 2] Preparation of hexaisobutyryl cercosporine
[0052]
[0053]The reaction formula of this example is shown above, and the specific reaction method is: mix cercosporin (20mg), isobutyric anhydride (500μL), magnesium powder (30mg), N,N-dimethyl-4-aminopyridine (50 mg) and a stirring bar were added into the reaction vessel, and reacted under nitrogen protection for 2 hours. After the reaction was completed, isobutyric anhydride (500 μL) was added again, and the reaction was continued for 2 hours. After the reaction was completed, 20 mL of dichloromethane was added to the reaction liquid, and then filtered, and the obtained filtrate was washed three times with 10 mL of distilled water. Finally, the organic phase was dried by adding anhydrous magnesium sulfate, and the filtrate was obtained by suction filtration, and concentrated using a rotary evaporator. Quickly separate by 200-300 mesh silica gel column chromatography, the eluent used is methanol / dichlorom...
Embodiment 3
[0056] [Example 3] Preparation of hexa-n-butyryl cercosporine
[0057]
[0058] The reaction formula of this example is shown above, and the specific reaction method is: cercosporin (20 mg), butyric anhydride (500 μL), magnesium powder (30 mg), N,N-dimethyl-4-aminopyridine ( 50 mg) and a stirring bar were added into the reaction vessel, and reacted for 2 hours under nitrogen protection. After the reaction was completed, butyric anhydride (500 μL) was added again, and the reaction was continued for 2 hours. After the reaction was completed, 20 mL of dichloromethane was added to the reaction liquid, and then filtered, and the obtained filtrate was washed three times with 10 mL of distilled water. Finally, the organic phase was dried by adding anhydrous magnesium sulfate, and the filtrate was obtained by suction filtration, and concentrated using a rotary evaporator. Quickly separate by 200-300 mesh silica gel column chromatography, the eluent used is methanol / dichloromethan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com