Method for simultaneously determining contents of pregabalin and hydroxybenzene ester bacteriostatic agent
A technique of pregabalin, determination method, applied in the directions of measuring devices, instruments, scientific instruments, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Embodiment 1: detection method example
[0080] 1. Experimental samples
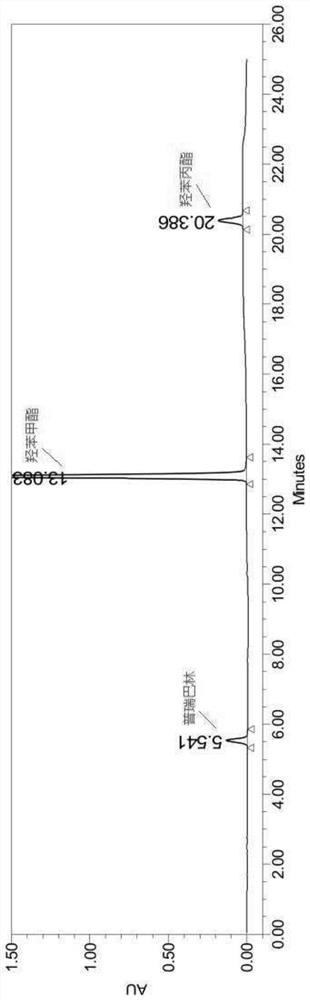
[0081] Sample stock solution: take appropriate amount of pregabalin, methyl paraben, and propyl paraben reference substance, weigh them accurately, and dilute them to contain about 20 mg of pregabalin, 130 μg of methyl paraben, and 15 μg of propyl paraben in each 1 ml. The solution, shake well, respectively as the reference substance stock solution; wherein, pregabalin diluted with pure water, paraben antibacterial agents diluted with methanol.
[0082] System suitability test solution: Take 1ml of each of the above stock solutions, put them in a 10ml measuring bottle, dilute to the mark with mobile phase A, shake well, and use it as a system suitability solution.
[0083] Blank solution: mobile phase A.
[0084] Pregabalin (EP, lot number: 2.0, purity 99.7%), methylparaben (Acstandard, lot number: 17528-01 purity 97.5%), propylparaben (Acstandard, lot number: A008525301-02 purity 99.3%).
[...
Embodiment 2
[0099] Example 2: Specificity Verification
[0100] Prepare blank auxiliary material solution (not containing pregabalin, hydroxyphenyl ester bacteriostatic agent), measure above-mentioned blank auxiliary material solution 1ml, dilute mobile phase A to 10ml, inject sample according to embodiment 1 chromatographic conditions, the result sees image 3 . Wherein the blank auxiliary material solution is an aqueous solution containing an appropriate amount of sodium dihydrogen phosphate, anhydrous disodium hydrogen phosphate, sucralose and strawberry essence.
[0101] figure 2 The peak positions of pregabalin and hydroxybenzoate antibacterial agents in figure 1 blank solvent and image 3 There is no chromatographic peak at the corresponding position of the auxiliary material solution, that is, there is no interference. Depend on Figure 1-Figure 3 It can be seen that the blank solvent and blank excipient solution did not interfere with the detection of the content of pregab...
Embodiment 3
[0102] Embodiment 3: linearity test
[0103] Pregabalin API (India Sun Pharmaceutical Co., Ltd., batch number: PRVNF18053), methylparaben (Jiangxi Alpha Hi-Tech Pharmaceutical Co., Ltd., batch number: 20180701), propylparaben (Hunan Erkang Pharmaceutical Co., Ltd., batch number : 11072010401)
[0104] Accurately weigh 2000.89mg of pregabalin bulk drug, put it in a 100ml volumetric flask, add purified water to dissolve and dilute to the mark, and use it as pregabalin stock solution; accurately weigh 130.42mg of methylparaben, put it in a 100ml volumetric flask, add Dissolve in methanol and dilute to the mark as the methyl paraben stock solution; accurately weigh 16.34 mg of the propyl paraben and put it in a 100ml volumetric flask, add methanol to dissolve and dilute to the mark as the propyl paraben stock solution. Accurately measure 1, 1.5, 2, 3, and 4 mL of pregabalin stock solution and place them in 20 mL volumetric flasks, respectively, add 1, 1.5, 2, 3, and 4 mL of hyd...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mobile phase a | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com