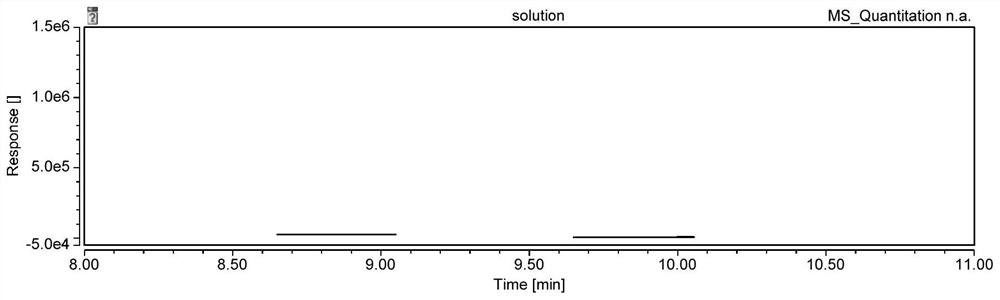
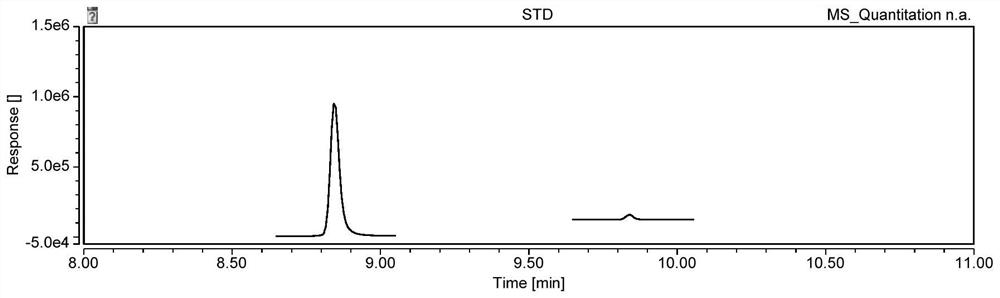
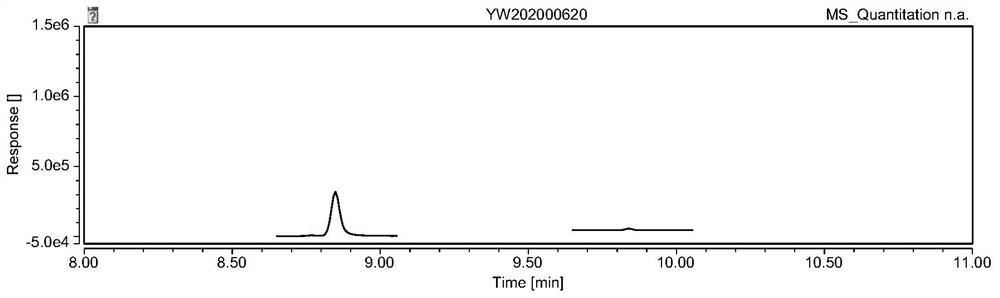
Analysis method of genotoxic impurity in moxifloxacin hydrochloride starting material
A technology of moxifloxacin hydrochloride and genotoxicity, which is applied in the directions of material separation, analysis materials, instruments, etc., can solve the problems such as no published reports on the determination method of genotoxic impurities, and achieves low detection cost, low toxicity, and improved work efficiency. The effect of efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The investigation of embodiment 1 derivatization temperature
[0048] 1. Sample pretreatment
[0049] The test product (100% limit spiked) solution: take moxifloxacin hydrochloride starting material 1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo -1 g of ethyl 3-quinolinecarboxylate, accurately weighed, put in a 50 ml measuring bottle, and accurately add the stock solution of impurity 2,4,5-trifluoro-3-methoxybenzoyl chloride (1.68 μg / ml) 2 ml, mix well, then precisely add 10ml of absolute ethanol, mix well. Prepare 3 copies in the same way, place them at 25°C, 30°C, and 35°C for 30 minutes, dissolve and dilute to the mark with dichloromethane, shake well, and obtain.
[0050] 2. Chromatographic conditions
[0051] Instrument: gas chromatography-mass spectrometry; chromatographic column: TG-5 ms (30 m × 0.25 mm × 0.25 μm); temperature program, the initial temperature is 100 ° C, maintained for 5 minutes, and the temperature is raised to 150 ° C at a rate of 1...
Embodiment 2
[0056] The investigation of embodiment 2 derivation time
[0057] 1. Sample pretreatment
[0058] The test product (100% limit spiked) solution: take moxifloxacin hydrochloride starting material 1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo -1 g of ethyl 3-quinolinecarboxylate, accurately weighed, put in a 50 ml measuring bottle, and accurately add the stock solution of impurity 2,4,5-trifluoro-3-methoxybenzoyl chloride (1.68 μg / ml) 2 ml, mix well, then precisely add 10ml of absolute ethanol, mix well. Prepare 3 parts in the same way, place them in a water bath at 30°C for 5 minutes, 10 minutes, and 15 minutes respectively, dissolve and dilute to the mark with dichloromethane, shake well, and obtain.
[0059] Determine according to the chromatographic conditions set in Example 1.
[0060] 2. Results and conclusions
[0061] Table 2 Screening results of derivation time
[0062] Derivation time 5 minutes 10 minutes 15 minutes Derivative peak a...
Embodiment 3
[0064] Example 3 Investigation of the volume-to-mass ratio of the derivatization reagent to the moxifloxacin hydrochloride starting material
[0065] 1. Sample pretreatment
[0066] The test product (100% limit spiked) solution: take moxifloxacin hydrochloride starting material 1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo - 1g of ethyl 3-quinolinecarboxylate, accurately weighed, put in a 50ml measuring bottle, and accurately add the stock solution of impurity 2,4,5-trifluoro-3-methoxybenzoyl chloride (1.68 μg / ml) 2ml, mix well, prepare 3 parts in the same way, then add 2ml, 5ml, 10ml of absolute ethanol respectively, and mix well. Heat in a water bath at 30°C for 10 minutes, dissolve and dilute to the mark with dichloromethane, shake well, and obtain.
[0067] The determination was carried out according to the chromatographic conditions determined in Example 1.
[0068] 2. Results and conclusions
[0069] Table 3 Screening results of derivatization reagent to sub...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com