Multi-terpyridyl metal organic ligand compound, five-membered flower ring-shaped supramolecule assembled thereby, and preparation
A ligand compound, metal-organic technology, applied in the field of new supramolecular synthesis, can solve the problems of no five-membered symmetrical flower-like supramolecular report, not very strict conservation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
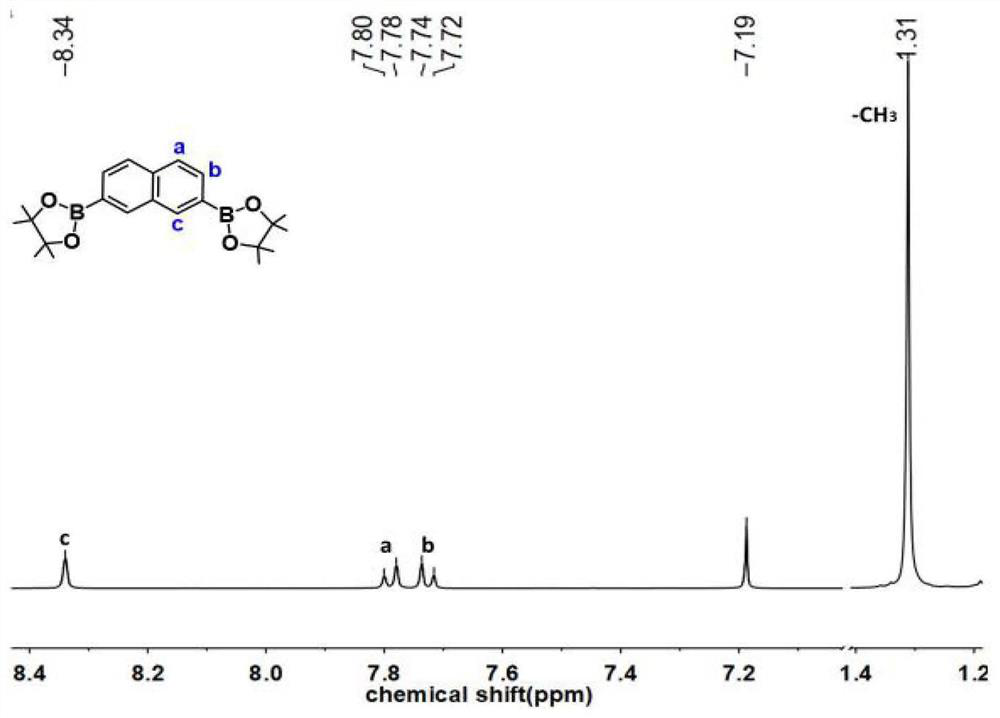
[0089] 2,7-diboronate naphthalene (S2):
[0090]
[0091] 2,7-dibromonaphthalene (500.0mg, 1.75mmol), biboronic acid pinacol ester (1.1g, 4.2mmol), potassium acetate (1.4g, 14.26mmol), catalyst [1,1'-bis(di Phenylphosphine)ferrocene]palladium dichloride (154.0mg, 0.21mmol), THF (15ml) were added into a 50mL round bottom flask, under nitrogen protection, heated and stirred at 85°C for 24 hours. After the reaction was completed, it was cooled to room temperature, extracted with water and dichloromethane, and the organic phase was collected and evaporated under reduced pressure to remove the solvent. Add a small amount of methanol and a large amount of petroleum ether for recrystallization, collect the liquid by filtration under reduced pressure, remove the solvent by rotary evaporation, add a small amount of methanol and then add a large amount of water, collect the solid by filtration under reduced pressure, and dry to obtain 565.0 mg of the product with a yield of 85%. NMR...
Embodiment 2
[0125] The supramolecule (five-membered rosette-shaped metal organic supramolecule) prepared in Example 1 was gelled. The five-member rosette-shaped organometallic supramolecule was dissolved in DMF at a concentration of 0.1 mmol / ml, and then left for 3-5 days to obtain a gel. For pictures of the gels described, see Figure 11 , for SEM characterization of the gel see Figure 12 . Figure 11 The medium glass bottle is inverted and the contents do not flow, indicating that it has successfully gelled; Figure 12 It shows that the molecules in the colloid are cross-linked to form a three-dimensional network structure.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com