Imidazole magnetic ionic liquid containing perylene bisimide structure, and preparation method and application of imidazole magnetic ionic liquid
A magnetic ionic liquid, perylene imide technology, applied in the application of biological imaging, imidazole magnetic ionic liquid, magnetic ionic liquid synthesis containing perylene imide structure, the application field of in vivo nuclear magnetic resonance imaging, can solve the problem Problems such as difficult compound application and poor solubility of PDI achieve good biocompatibility, high atom utilization and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] The synthesis of intermediate product a, its chemical structure is as follows:
[0061]
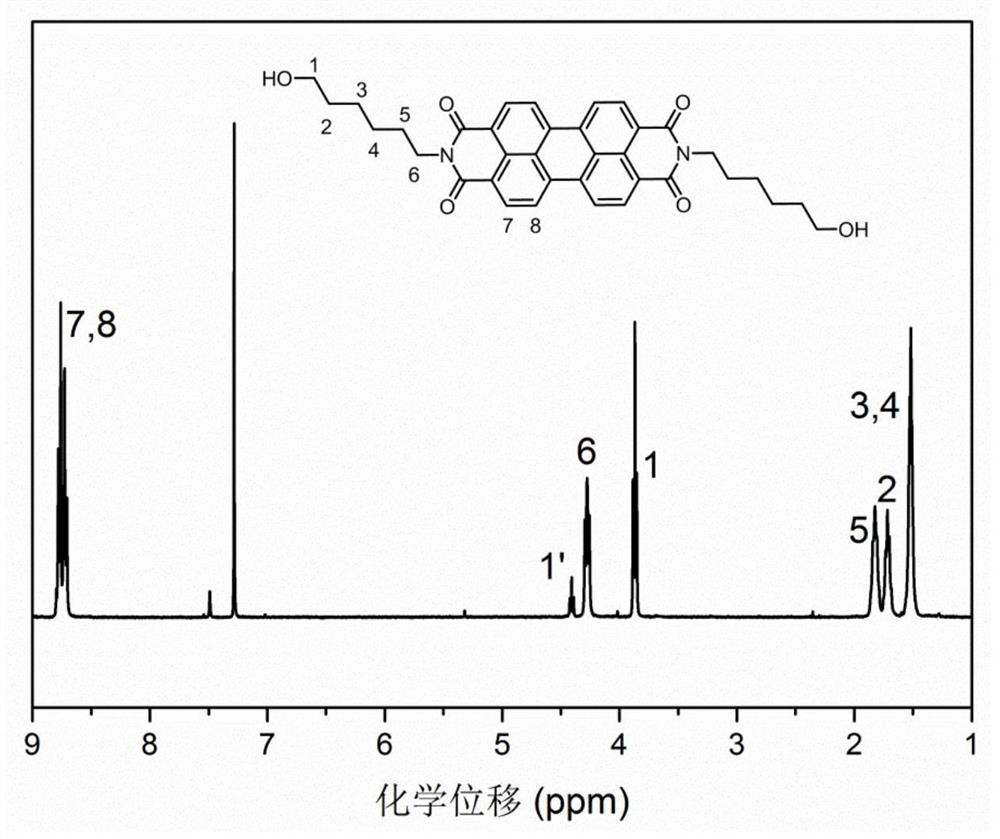
[0062] Weigh 3,4,9,10-perylenetetracarboxylic dianhydride (5mmol, 1.9616g), 6-amino-1-hexanol (20mmol, 2.3438g) and imidazole (20g), place in an oil bath and keep the reaction at React at 150°C for 36h. After the reaction was completed, the product was washed with excess acetone several times, and after vacuum drying for 24 h, a purple-black solid product was obtained. Yield: 98%. figure 1 is the NMR spectrum of a ( 1 H-NMR, 400Hz, CDCl 3 ).
[0063] 1 H NMR (400MHz, CDCl 3 )δ: 8.84–8.75 (multiplet, hydrogen Ar-H on the benzene ring), 4.42 (triplet), 3.87 (triplet), hydrogen-CH of the methylene group connected to the hydroxyl group 2 -OH, 4.29 (triplet, hydrogen N-CH of methylene connected to nitrogen element 2 -), 1.89-1.77 (multiple peaks, the hydrogen N-CH on the methylene separated from the nitrogen element by a methylene 2 -CH 2 -), 1.76–1.68 (multiple peaks, hydr...
Embodiment 2
[0065] The synthesis of intermediate product b, its chemical structure is shown below:
[0066]
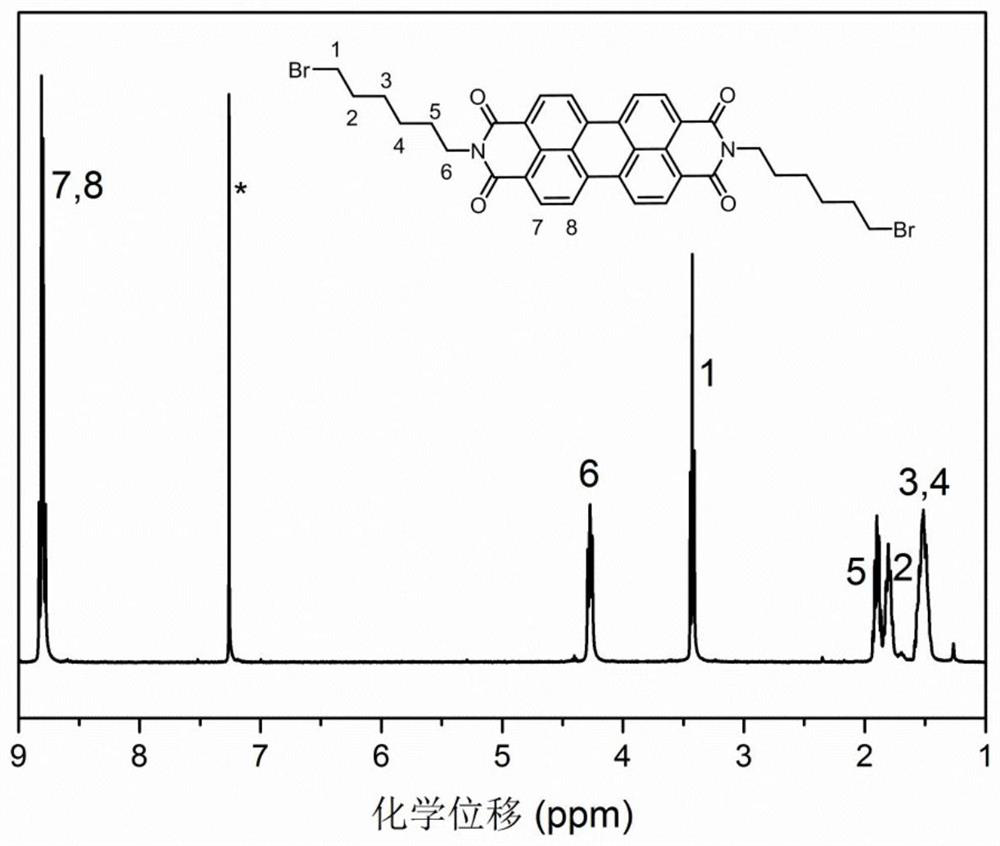
[0067] The intermediate product a (4mmol, 2.3627g) was weighed and added to 40mL of toluene, and an aqueous solution of hydrogen bromide of 8 times the number of moles was added to the suspension. The reaction was heated to reflux and maintained at reflux for 36h, and the reaction temperature was 120 degrees Celsius. After the reaction was completed, the reaction was cooled to room temperature, and the product was washed with acetone for several precipitations. After vacuum drying for 24 h, the product was obtained as a purple-red solid. Yield: 94%. figure 2 is the NMR spectrum of b ( 1 H-NMR, 400Hz, CDCl 3 ).
[0068] 1 H NMR (400MHz, CDCl 3 )δ: 8.81 (quartet, hydrogen Ar-H on the benzene ring), 4.28 (triplet, hydrogen-CH of methylene connected to bromine element 2 -Br), 3.43 (triplet, hydrogen N-CH of methylene connected to nitrogen element 2 -), 1.97 – 1.86 (multip...
Embodiment 3
[0070] The synthesis of intermediate product c, its chemical structure is shown below:
[0071]
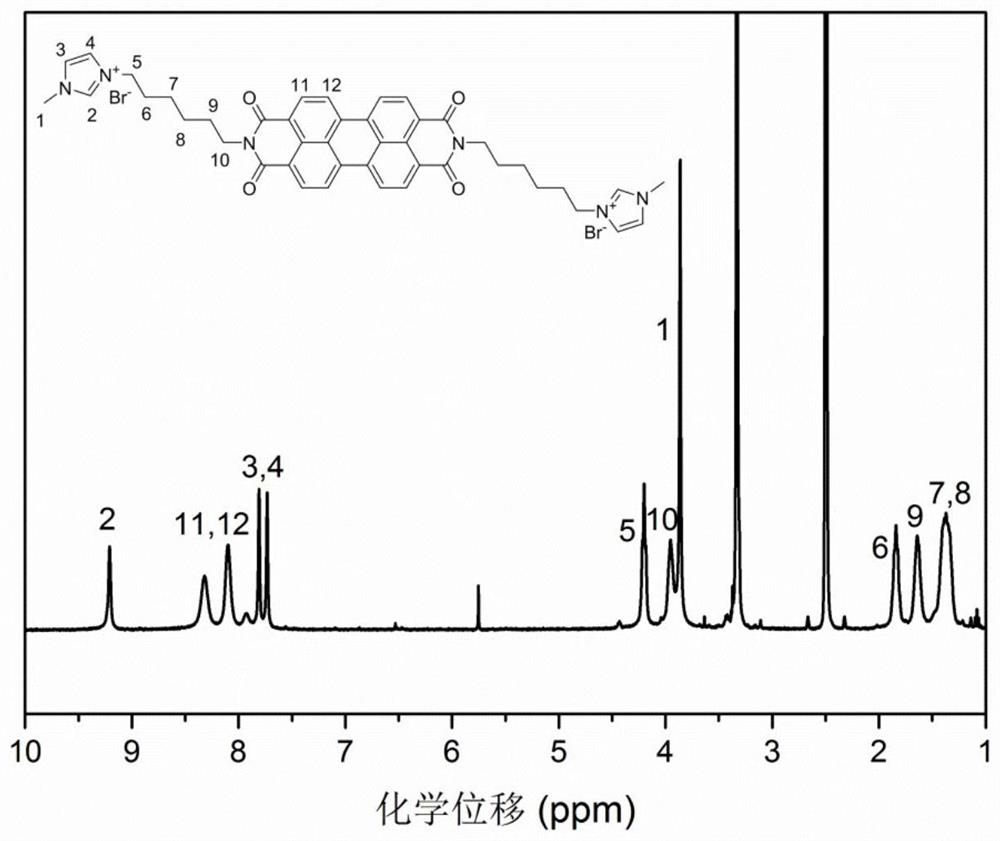
[0072] Intermediate product b (2mmol, 1.4329g) was weighed and added to a 25mL round bottom flask, 1-methylimidazole (40mmol, 3.2mL) was added to the flask and 5mL of acetone was added. Place in a water bath and keep the reaction at 90°C for 24 hours. After the reaction was completed, the solvent was removed under reduced pressure by a rotary evaporator, and the crude product was precipitated with diethyl ether several times. After vacuum drying for 24 h, the product was obtained as a purple solid. Yield: 100%. image 3 is the NMR spectrum of c ( 1 H-NMR, 400Hz, DMSO-d 6 ).
[0073] 1 H NMR (400MHz, DMSO-d 6 )δ: 9.21 (singlet, hydrogen N-CH-N on the imidazole ring), 8.45-7.89 (multiplet, hydrogen Ar-H on the benzene ring), 7.82 (singlet, hydrogen N on the imidazole ring -CH-CH-N), 7.74 (singlet, hydrogen N-CH-CH-N on the imidazole ring), 4.21 (triplet, and imidazole rin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com