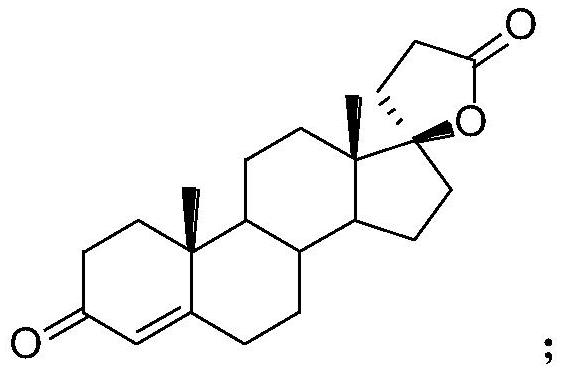
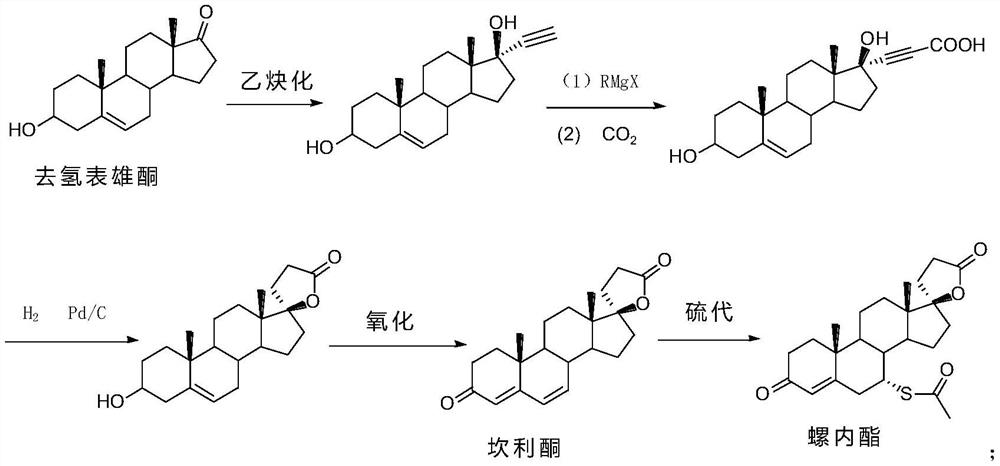
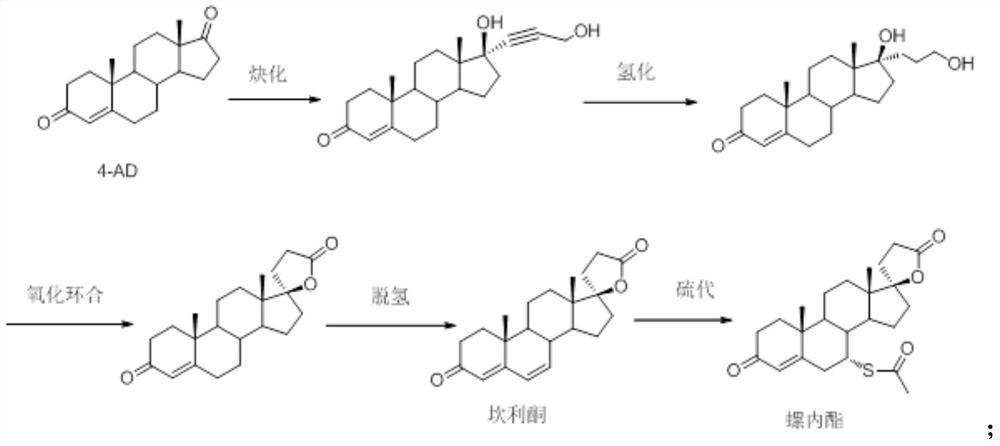
Synthesis process of steroid compound, canrenone and spirolactone
A technology for steroid compound and synthesis process, which is applied in the field of drug synthesis, can solve the problems of poor appearance and quality of canrenone and spironolactone, difficulty in solvent recovery, and high COD in waste water, and achieves a process that is environmentally friendly, high in molar yield, and waste water. small amount of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] This embodiment provides a synthetic process for steroidal compounds, and the specific synthetic route is as follows:
[0101]
[0102] Wherein the compound shown in the formula I can be purchased from the market.
[0103] 100g of ethanol, 15.6g of sodium ethoxide, and 48.9g of diethyl malonate were added to a three-necked flask, then 50g of the compound of formula I was added, the temperature was raised to 30°C and the reaction was kept for 6 hours, and then 200ml of 5% sodium hydroxide aqueous solution was added, React at 0°C for 8 hours, add 300ml of 10% hydrochloric acid aqueous solution, react at 0°C for 24 hours, cool down, filter with suction to obtain the compound shown in formula II (R=H), and recrystallize from ethanol to obtain 54.2g of white crystals.
[0104] The relevant characterization data of the compound of formula II are as follows:
[0105] [α] 20 D =+158°(C=1,CH 2 Cl 2 ).
[0106] UVmax: 240nm.
[0107] Elemental Analysis: Theoretical Calc...
Embodiment 2
[0115]
[0116] Add 100g of ethanol, 22g of sodium ethoxide, and 62.1g of diethyl malonate into a three-necked flask, then add 50g of the compound of formula I, raise the temperature to 60°C and keep it warm for 4 hours, then add 200ml of 10% hydrochloric acid aqueous solution, and react at 60°C 0.5 hour, cooling, suction filtration, obtains the compound shown in formula II (R=C 2 h 5 ), recrystallized from ethanol to obtain 57.6 g of white crystals.
Embodiment 3
[0118]
[0119] Add 100g of ethanol, 24g of sodium ethoxide, and 55.6g of methyl ethyl malonate into a three-necked flask, then add 50g of the compound of formula I, heat up to 80°C for 6 hours, and then add 200ml of 5% aqueous sodium hydroxide solution. React at 25°C for 8 hours, add 300ml of 10% hydrochloric acid aqueous solution, heat up to 60°C and react for 0.5 hours, cool down, filter with suction to obtain the compound shown in formula II (R=H), and recrystallize from ethanol to obtain 54.4g of white crystals.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com