Method for identifying amino-containing metabolite isomers through direct mass spectrometry and application of amino-containing metabolite isomers
A technology for isomers and metabolites, applied in the field of amino metabolite isomer identification, can solve the problems of complex processing, cumbersome operation, inability to distinguish isomers, etc., and achieves high accuracy, simple operation, good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The steps of the detection method for the identification of amino acid isomers are as follows:
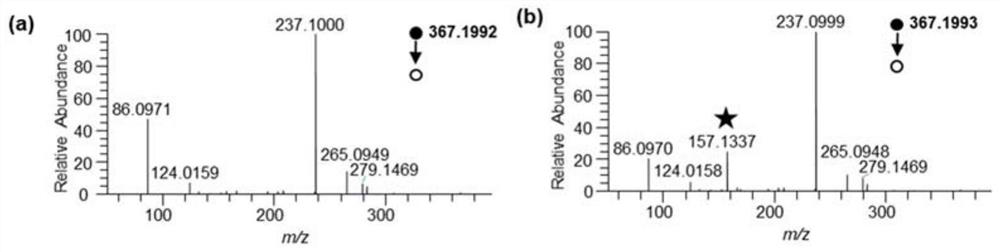
[0043] (1) Derivatization reaction: prepare 100 μL of 50 mM sodium bicarbonate solution of 10 mM leucine, isoleucine, alanine, β-alanine, aspartic acid and iminodiacetic acid standards respectively, pH About 8, add 40μL 50mM DIPP-L-Ala-NHS acetonitrile solution under ice bath conditions, vortex mix each solution, react in ice bath for more than 10min, and then react for more than 10min at room temperature; vacuum the obtained mixture Remove acetonitrile with a centrifugal evaporation concentrator; add 10% formic acid solution to the residue to adjust the pH to 2-3; then use a reversed-phase Vac C18 desalting, collect the eluate and remove acetonitrile with a vacuum centrifugal evaporation concentrator, freeze-dry the obtained solid to obtain the amino metabolite derivatized product, add 5% acetonitrile to the obtained amino metabolite derivatized product to resuspend, Fina...
Embodiment 2
[0052] Example 2, the identification steps of amino-containing metabolite isomers in cells are as follows:
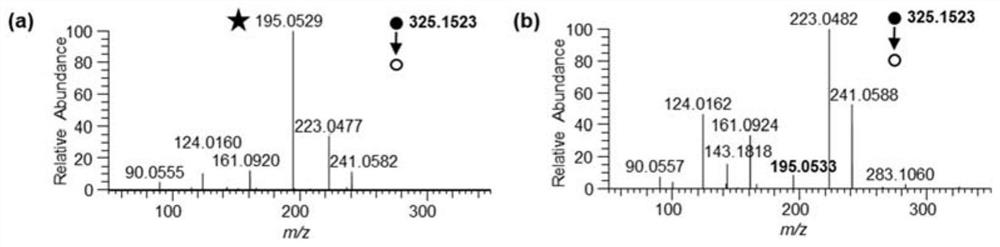
[0053] Step (1) Extraction of cell metabolites: take the cultured HepG2 cells out of the incubator, and aspirate the medium; wash the cells with 2 mL of 37°C PBS to remove the residual medium, then aspirate the PBS, and repeat this step twice; Then scrape the cells into a 15mL centrifuge tube, wash the culture plate with 1mL-20°C methanol, and put the washed methanol into the 15mL centrifuge tube; put the centrifuge tube into liquid nitrogen and freeze for 30s. Take out the centrifuge tube from liquid nitrogen, thaw on ice for 2 min, vortex for 15 s, sonicate for 30 s, incubate on ice for 10 min; vortex for 15 s, centrifuge at 3000 g for 5 min, and collect the supernatant. Add 500 μL methanol to the precipitate, repeat the above extraction steps, and collect the supernatant; then add 500 μL ultrapure water to the precipitate, and collect the supernatant according to the...
Embodiment 3
[0058] Example 3, the identification steps of amino-containing metabolite isomers in animal tissues are as follows:
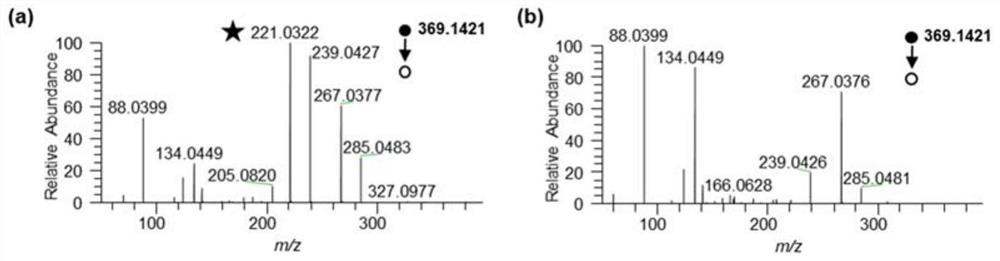
[0059] Step (1) Extraction of tissue metabolites: Methanol / chloroform / water was formulated as an extractant in a ratio of 2:2:3. Weigh 10 mg of mouse liver tissue sample, and homogenize it in a mixed solution of 4 mL / g methanol and 2 mL / g water at 4°C. The homogenate was transferred into a small glass bottle, and 4 mL / g chloroform and 4 mL / g water were added and vortexed for 60 s. After standing on ice for 15 minutes, centrifuge at 10,000 rpm for 10 minutes at 4°C, take the supernatant and transfer it to a glass bottle, remove the methanol and water from the obtained liquid, and store the tissue metabolite powder in a -80°C refrigerator.
[0060] Step (2) Derivatization reaction of tissue metabolites: Add 100 μL of 50 mM NaHCO to 10 mg of tissue-extracted metabolite powder 3 After the solution was fully dissolved, it was centrifuged at 15 000 g for 15 min, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com