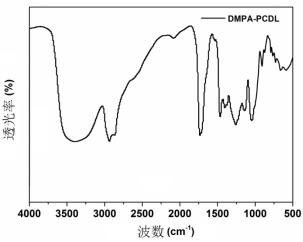
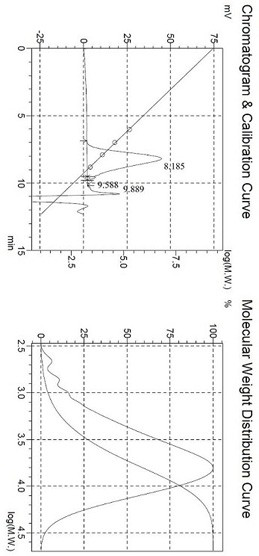
Preparation method of hydrophilic polycarbonate diol
A technology of polycarbonate diols and diols, which is applied in the field of preparation of hydrophilic polycarbonate diols, can solve problems such as difficult removal, residual polyurethane monomers, and affecting the performance of water-based polyurethanes, so as to improve hydrophilicity High purity, high purity, and controllable product molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047]Under the protection of nitrogen, 124 g of diethyl carbonate, 82 g of 1,4-butanediol, and 13.4 g of dimethylolpropionic acid (DMPA) were added to a 500 mL four-necked flask, and the catalyst 0.012 Put 57 g of tetrabutyl titanate and 57 g of isooctane entrainer into an oil bath, put a rectification and fractionation device equipped with fillers on the neck of the bottle, and carry out atmospheric rectification at 110 ° C. After reacting for 5 hours, the by-products formed by the reaction and isooctane formed an azeotrope and flowed out. After the fraction was removed to the theoretical value, the temperature continued to rise to 180 °C; the vacuum distillation operation was carried out in stages. Set the vacuum degree to 3 kPa, continue to vacuumize for 1 h; then continue to reduce the pressure, set the vacuum degree to 100 Pa, and react for 1 h; stop the reaction after the product reaches the ideal viscosity (8000~16000 mPa.s), and wait for the system to cool to After ro...
Embodiment 2
[0052] Under the protection of nitrogen, 94.5 g of dimethyl carbonate, 82 g of 1,4-butanediol, and 13.4 g of dimethylolpropionic acid (DMPA) were added to a 500 mL four-necked flask, and the catalyst 0.012 g of tetrabutyl titanate and 57 g of isooctane entrainer were put into an oil bath, and a rectification and fractionation device equipped with fillers was placed on the neck of the bottle, and the atmospheric rectification reaction was carried out at 90°C. After reacting for 6 hours, the by-products formed by the reaction and isooctane formed an azeotrope and flowed out. After the fraction was removed to the theoretical value, the temperature continued to rise to 170 °C, and the vacuum distillation operation was carried out in stages. After replacing the vacuum device, the vacuum degree Set it to 3 kPa, continue vacuuming and reacting for 1 hour, then continue to reduce pressure, set the vacuum degree to 100 Pa, react for 1 hour, stop the reaction after the product reaches th...
Embodiment 3
[0055] Under the protection of nitrogen, 124 g of diethyl carbonate, 82 g of 1,4-butanediol, and 14.8 g of dimethylolbutyric acid (DMBA) were added to a 500 mL four-necked flask, and the catalyst 0.016 g of triethylamine and 100 g of n-heptane entrainer are placed in an oil bath, and a rectification and fractionation device equipped with fillers is placed on the neck of the bottle, and the atmospheric rectification reaction is carried out at 110 ° C. Reaction 6 h, the by-products formed by the reaction and isooctane form an azeotrope and flow out. After the fraction is removed to the theoretical value, continue to heat up to 180 °C, and carry out vacuum distillation operations in stages. After replacing the decompression device, set the vacuum degree to to 3 kPa, continue the vacuum reaction for 1 h, then continue to reduce the pressure, set the vacuum degree to 100 Pa, and react for 1 h. After the product reaches the ideal viscosity (7000~16000mPa.s), the reaction is stopped, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com