Fusion protein modified by klebsiella pneumonia polysaccharide, and application of fusion protein
A technology for Klebsiella and pneumonia, applied in the field of biomedicine, can solve the problems of difficult vaccines, high cost, and few applications in the field of bacteria, and achieve the effect of improving immune effect and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
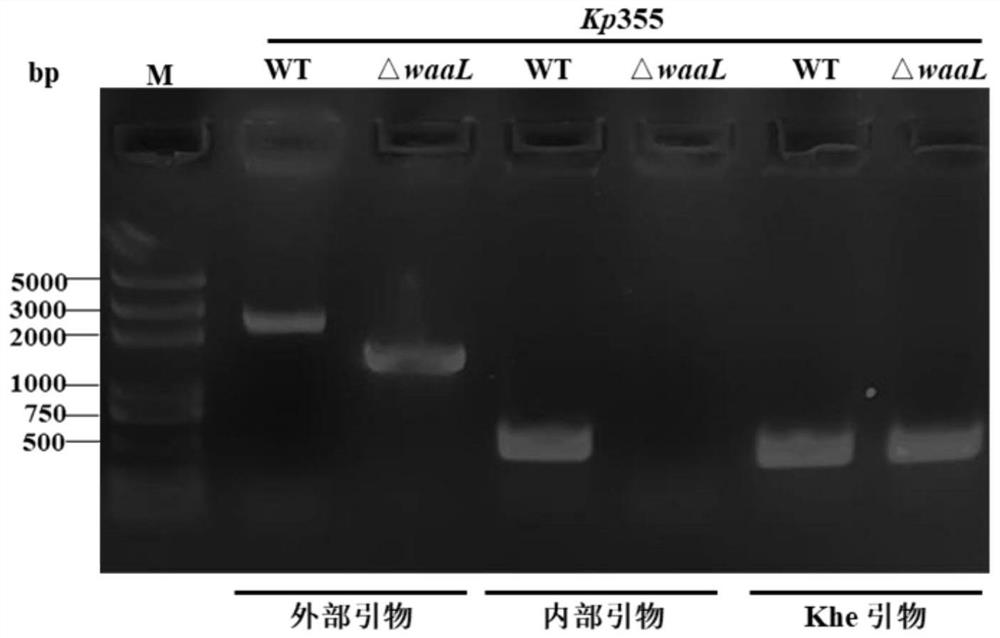
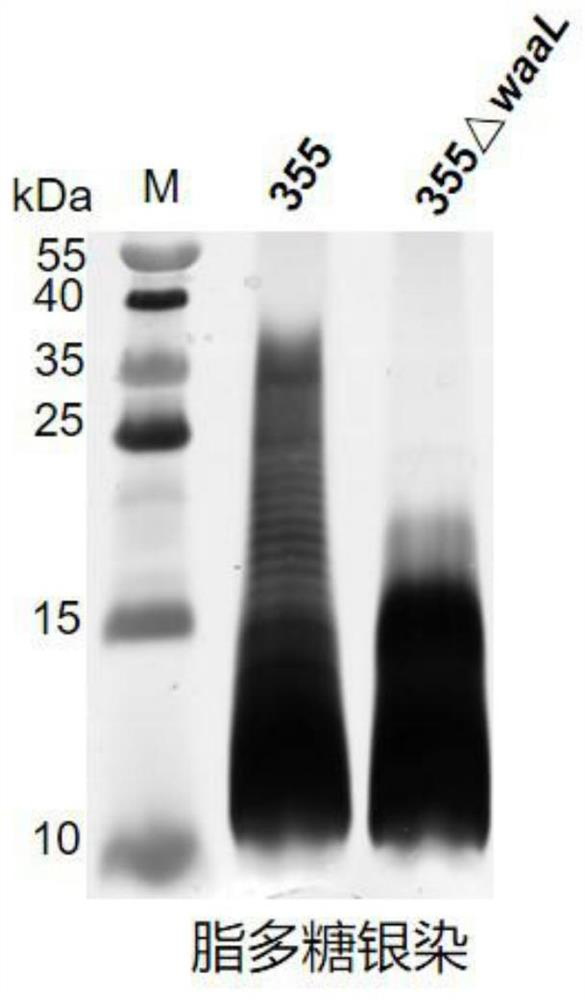
[0075] Example 1, Klebsiella pneumoniae with lipopolysaccharide synthesis deficiency
[0076] 1. Preparation of Klebsiella pneumoniae 355 strains (also known as KP355 or 355) with deletion of O-antigen ligase (waaL) gene
[0077] 1. Preparation of linear targeting DNA fragments
[0078] 1) Design of PCR primers
[0079] Compare the waaL gene of Klebsiella pneumoniae on NCBI with the local Blast database of the whole genome of 355 strains of Klebsiella pneumoniae, search for the waaL homologous gene on the genome of 355 strains, and then based on the gene and its upstream and downstream sequences , using Primer 6.0 software to complete the design of relevant primers (see Table 1 for details). A pair of primers, waaL-up-F / waaL-up-R and waaL-dw-F / waaL-dw-R, were designed respectively at the upstream (5' end) and downstream (3' end) of the waaL gene. For the convenience of operation, restriction enzyme cutting sites BamHI and SalI will be added to the end of the primer of the u...
Embodiment 2
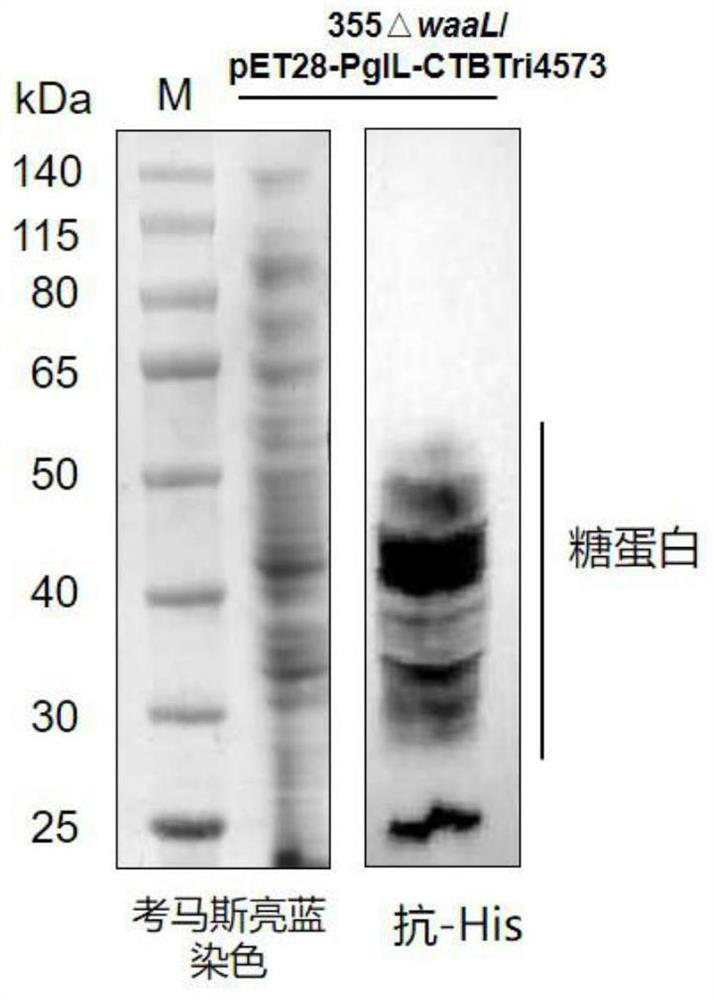
[0110] Preparation of Example 2 Nano Glycoprotein Particles CTBTri4573-OPS
[0111] 1) Construction of the expression vector of the glycosylated fusion protein CTBTri4573
[0112] Construction of Neisseria meningitidis glycosyltransferase PglL expression vector: The amino acid sequence of Neisseria meningitidis glycosyltransferase PglL (GeneBank: JN200826.1) is shown in SEQ ID No.1, and its coding sequence is shown in SEQ ID No. 180-1994 nucleotides of .2. The 1st-6th nucleotide of SEQ ID No.2 is the XbaI recognition site, and the 105th-2240th nucleotide of SEQ IDNo.2 is the sequence of the PglL expression cassette. In the PglL expression cassette, the expression of PglL is controlled by tac The promoter is activated, and the expression cassette is named tacpglL. Wherein, the 105th-133rd nucleotide of SEQ ID No.2 is the sequence of the tac promoter, the 180th-1994th nucleotide is the coding sequence of Neisseria meningitidis glycosyltransferase PglL, and the 2475-2480th The...
Embodiment 3
[0130] The preparation of embodiment 3CTB4573-OPS
[0131] 1) Construction of the expression vector of the glycosylated carrier protein CTB4573
[0132] The encoded amino acid sequence of CTB4573 is shown in SEQ ID No.5, the gene sequence is shown in 178-654 of SEQ ID No.6, and the 1-6 nucleotides of SEQ ID No.6 are XbaI recognition sites, The 103-131 nucleotides are the sequence of the tac promoter, the 178-630 nucleotides are the coding sequence of CTB4573, the 631-636 nucleotides are the XhoI recognition sequence, and the 637-654 nucleotides are His tag. In the sequence, the CTB4573 sequence is a glycosylation modification sequence, and it is a peptide segment consisting of 29 amino acids at positions 45-73 of pili protein PilE.
[0133] Digest the DNA molecule shown in SEQ ID No.6 with XbaI and XhoI to obtain a gene fragment; digest pET28a(+) with XbaI and XhoI to obtain a large vector fragment; connect the gene fragment to the large vector fragment to obtain a recombina...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com