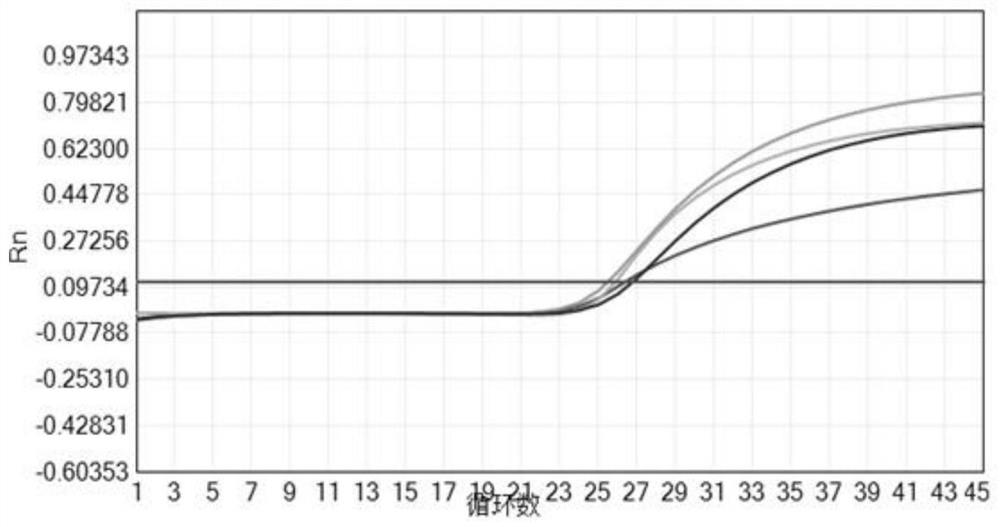
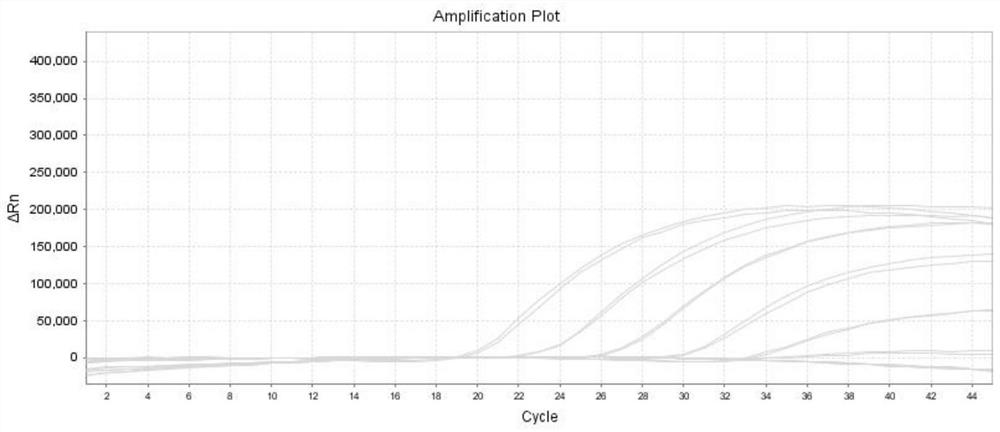
Multiplex real-time PCR kit and method for detecting respiratory pathogens and application
A kit and pathogen technology, applied in the field of biomedicine, can solve the problems of the narrow detection range of respiratory virus nucleic acid detection kits, the detection throughput is limited by the instrument, and the detection cycle is prolonged, so as to shorten the detection time, reduce the detection cost, and improve the detection efficiency. wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] This embodiment provides a real-time PCR multiplex detection kit for detecting influenza A virus, influenza B virus, human rhinovirus and human metapneumovirus, including probes for detecting influenza A virus and specific Primer pairs for the selective amplification of influenza A virus target genes, probes for detection of influenza B virus and primer pairs for specific amplification of influenza B virus target genes, probes for detection of human rhinovirus and specificity Primer pair for amplifying human rhinovirus target gene, probe for detection of human metapneumovirus and primer pair for specific amplification of human metapneumovirus target gene, MgCl 2 , dNTPsMixture, AMV reverse transcriptase, RNase inhibitor, Taq DNA polymerase, plasmid mixture containing the target gene and nuclease-free water;
[0053] This embodiment provides a method for detecting bronchial lavage fluid using the above kit:
[0054] 1. Extract the nucleic acid of bronchial lavage fluid ...
Embodiment 2
[0070] This embodiment is the same as Example 1, except that the clinical samples, the primers and probe concentrations amplified by each pathogenic microorganism, the system component concentration of the amplification reaction, and the conditions of the amplification reaction are different from Example 1: this implementation The clinical sample taken for example is sputum, the primer concentration of each pathogenic microorganism amplification is 0.1 μ M, and the probe concentration is 50 nM; the MgCl in the reaction buffer described in the amplification reaction system 2 The working concentration of dNTPs Mixture is 2mM, the working concentration of dNTPs Mixture is 350μM, the working concentration of AMV reverse transcriptase in the enzyme mixture is 4U / μL, the working concentration of RNase inhibitor is 35U / μL, and the working concentration of TaqDNA polymerase 4U / μL, the amplification conditions are shown in the table below:
[0071]
[0072] In this example, the CT v...
Embodiment 3
[0074]This embodiment is the same as Example 1, except that the clinical samples, the primers and probe concentrations amplified by each pathogenic microorganism, the concentration of components in the amplification reaction system and the conditions of the amplification reaction are different from Example 1: this implementation The clinical sample that example takes is alveolar lavage fluid, and the primer concentration that each pathogenic microorganism is amplified is 1.0 μ M, and probe concentration is 250 nM; 2 The working concentration of dNTPs Mixture is 4mM, the working concentration of dNTPs Mixture is 450μM, the working concentration of AMV reverse transcriptase in the enzyme mixture is 6U / μL, the working concentration of RNase inhibitor is 45U / μL, the working concentration of Taq DNA polymerase The concentration is 6U / μL; the amplification conditions are shown in the table below:
[0075]
[0076] In this example, the CT value of human rhinovirus is ≤40, and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com