Test strip for detecting CD2v and MGF360 mucous membrane antibodies of African swine fever viruses and application of test strip
A technology of African swine fever virus and African swine fever, which is applied to test strips for detecting African swine fever virus CD2v and MGF360 mucosal antibodies and its application field, which can solve the problem of difficult real-time detection, low sensitivity and high cost in grassroots farms and other problems, to achieve the effect of high uniformity, high sensitivity and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
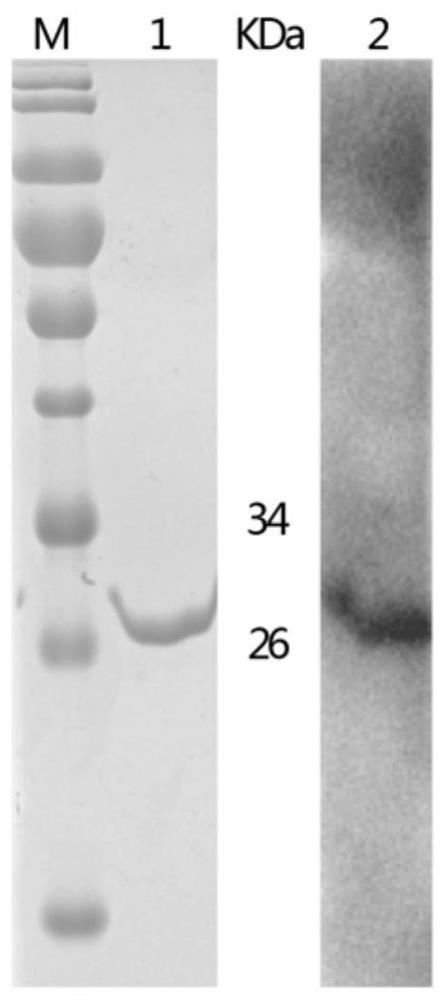
[0038] Embodiment 1 Preparation of recombinant protein CD2v and recombinant protein MGF360
[0039] 1. Construction of recombinant bacteria for preparing recombinant protein CD2v
[0040] Referring to the complete gene sequence of the first African swine fever SY18 in my country (MH766894, EP402R, 73369-74451) published by the NCBI database, the gene of the recombinant protein CD2v was designed. The sequence is shown in SEQ ID NO: 1. The amino acid sequence of the recombinant protein CD2v is shown in Shown in SEQ ID NO:2. The gene of recombinant protein CD2v was obtained by gene synthesis (provided by Nanjing KingScript Biotechnology Co., Ltd.), and the gene of recombinant protein CD2v was inserted between EcoRI and HindIII of the polyclonal restriction site of vector pET-32a(+) , to obtain the recombinant plasmid pET-32a-CD2v.
[0041] By "heat shock" method, the recombinant plasmid pET-32a-CD2v was transformed into E.coli BL21(DE3) competent cells to obtain the recombinant ...
Embodiment 2
[0049] Embodiment 2 Preparation of sample diluent of the present invention
[0050] The sample diluent of the present invention: contains the African swine fever virus double protein-marker. The African swine fever virus dual protein-marker is a mixture of recombinant protein CD2v labeled with quantum dot microspheres and recombinant protein MGF360 labeled with quantum dot microspheres.
[0051] The preparation method of sample diluent of the present invention comprises the steps:
[0052] (1) Preparation of recombinant protein CD2v labeled with quantum dot microspheres
[0053] Quantum dot microsphere suspension with a concentration of 1.2 μg / μL (purchased from Beijing Najing Biotechnology Co., Ltd., product number FM610C, hydrated particle size is 120nm) and MES buffer with a concentration of 0.02M and pH 7.0 were mixed by volume. Mix at a ratio of 1:1, then add EDC (1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride) and 10 mg / mL NHS (N -Hydroxysuccinimide) and ...
Embodiment 3
[0060] The preparation of embodiment 3 test strips of the present invention
[0061] Such as Figure 3-5 , the test strip for detecting African swine fever virus CD2v and MGF360 mucosal antibodies includes a bottom plate 2, on which a sample pad 3, a nitrocellulose membrane 4 and an absorbent pad 7 are sequentially arranged. A test line 5 and a quality control line 6 are arranged on the nitrocellulose membrane 4, and the test line 5 is set near the side of the sample pad, and the test line is coated with a mouse anti-pig SC protein monoclonal antibody; the quality control line 6 is close to the water-absorbing pad 7 One side is set, and the quality control line is coated with purified protein of African swine fever positive serum.
[0062] 1. Preparation of purified protein from African swine fever positive serum
[0063] (1) Sample preparation
[0064]Recombinant protein CD2v and recombinant protein MGF360 were used as immunogens, and adult New Zealand white rabbits (1-2Kg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com