GSH concentration responsive pharmacosome for treating hyperthyroidism and preparation method
A technology of thyroid function and responsiveness, which is applied in the direction of pharmaceutical formulations, liposome delivery, and medical preparations of non-active ingredients. It can solve the problems of short biological half-life, large oral doses of drugs, and frequent taking times to achieve enhanced penetration. Sexuality, prolonged action time, and the effect of optimal drug selection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
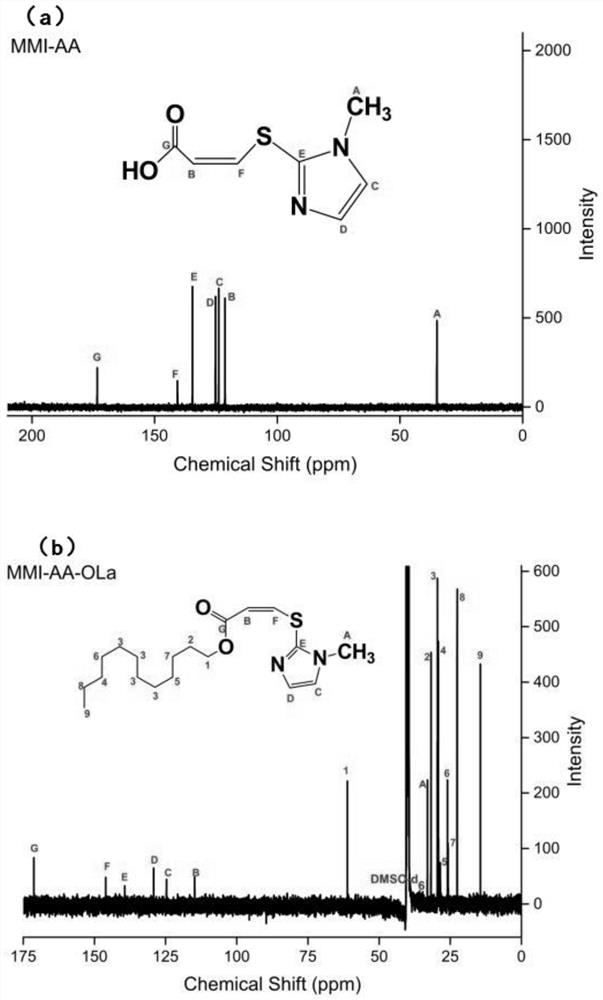
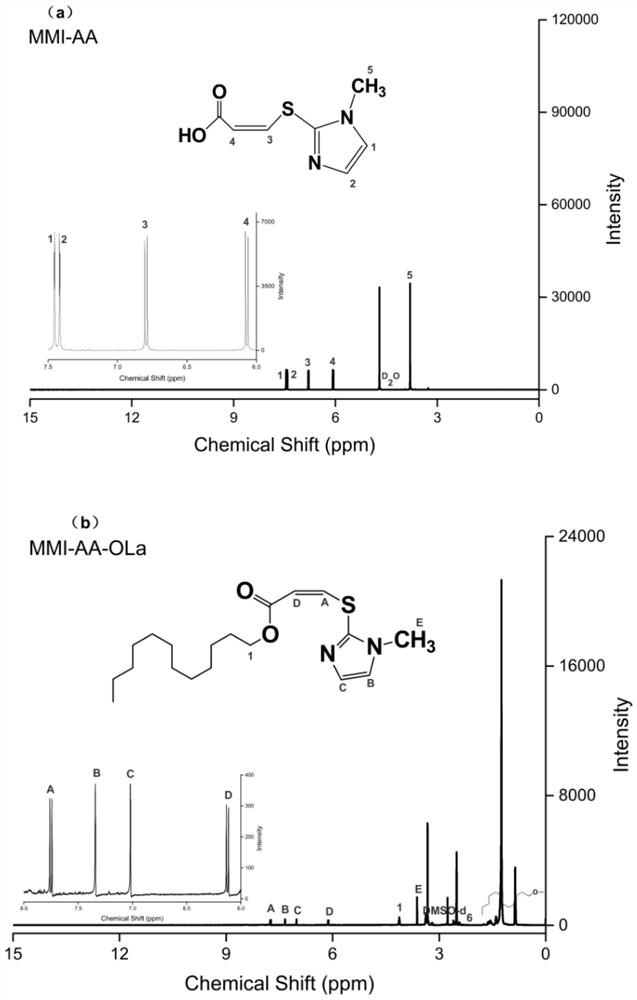
[0033] (1) Preparation of 3-[(1-methyl-1H-imidazol-2-yl)thio]-2-acrylic acid (methimazole acrylic acid, MMI-AA)
[0034] Methimazole (10mmol) was dissolved in methanol (1mol), sodium methoxide (10mmol) was added thereto under stirring until the methimazole was completely dissolved, and then propiolic acid (100mmol) was added therein. Reaction temperature: 10°C, under nitrogen protection, and react under reflux for 200 hours. After the reaction, use a rotary evaporator to rotate the reaction solution at 32°C for 10 minutes to obtain a thick white liquid; freeze-dry the thick white liquid for 10 hours, and obtain MMI-AA (white flake solid) after lyophilization .
[0035] (2) Preparation of 3-[(1-methyl-1H-imidazol-2-yl)thio]-2-propenoic acid-ethyl ester (methimazole ethyl acrylate, MMI-AA-OEt)
[0036]The obtained methimazole acrylic acid (1 mmol) and EDC (6 mmol) were dissolved in DMF (129 mmol), activated under stirring for 20 min, and NHS (15 mmol) was added after 20 min to...
Embodiment 2
[0038] (1) Preparation of 3-[(1-methyl-1H-imidazol-2-yl)thio]-2-acrylic acid (methimazole acrylic acid, MMI-AA)
[0039] Methimazole (10 mmol) was dissolved in methanol (0.8 mol), sodium methoxide (10 mmol) was added thereto under stirring until the methimazole was completely dissolved, and then propiolic acid (10 mmol) was added therein. Reaction temperature: 20°C, under nitrogen protection, under reflux for 150 hours. After the reaction, use a rotary evaporator to rotate the reaction solution at 32°C for 20 minutes to obtain a thick white liquid; freeze-dry the thick white liquid for 30 hours, and obtain MMI-AA (white flake solid) after lyophilization .
[0040] (2) Preparation of 3-[(1-methyl-1H-imidazol-2-yl)thio]-2-acrylic acid-n-octyl ester (methimazole n-octyl acrylate, MMI-AA-OCa)
[0041] The obtained methimazole acrylic acid (10mmol) and EDC (10mmol) were dissolved in DMF (129mmol), activated under stirring for 20min, and NHS (10mmol) was added after 20min to stabi...
Embodiment 3
[0043] (1) Preparation of 3-[(1-methyl-1H-imidazol-2-yl)thio]-2-acrylic acid (methimazole acrylic acid, MMI-AA)
[0044] Methimazole (30mmol) was dissolved in methanol (0.5mol), sodium methoxide (10mmol) was added thereto under stirring until the methimazole was completely dissolved, and then propiolic acid (10mmol) was added therein. Reaction temperature: 50°C, under nitrogen protection, and react under reflux for 100 hours. After the reaction, use a rotary evaporator to evaporate the reaction solution at 32°C for 60 minutes to obtain a thick white liquid; freeze-dry the thick white liquid for 50 hours, and obtain MMI-AA (white flake solid) after lyophilization .
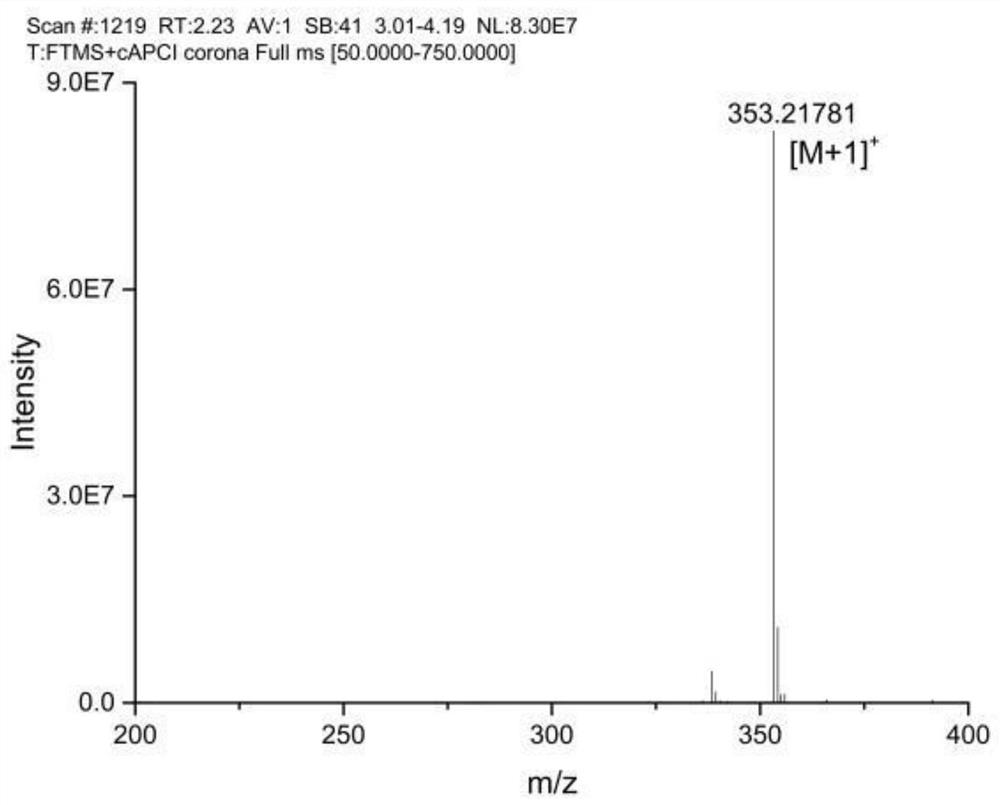
[0045] (2) Preparation of 3-[(1-methyl-1H-imidazol-2-yl)thio]-2-acrylic acid-dodecyl (methimazole acrylate dodecyl, MMI-AA-OLa)
[0046] Dissolve methimazole acrylic acid (9mmol) and EDC (27mmol) in DMF (260mmol) and activate for 20min with stirring. After 20min, add NHS (45mmol) and stabilize for 1 hour with sti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com